Chemistry. 2018 Aug 6;24(44):11426-11432. doi: 10.1002/chem.201802032
Simon M, Ali LMA, El Cheikh K, Aguesseau J, Gary-Bobo M, Garcia M, Morère A, Maillard LT.
Abstract
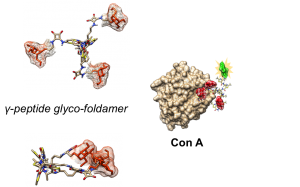
Sugars play key roles in many molecular and cellular communication processes involving a family of proteins named lectins. The low affinity associated with sugar recognition is generally counterbalanced by the multivalent nature of the interaction. While many polyglycosylated architectures have been described, only a few studies focused on the impact of topology variations of the multivalent structures on the interaction with lectin proteins. One major interest of our group concerns the design of new highly predictable and stable molecular pseudo‐peptide architectures for therapeutic applications. In such a context, we described a class of constrained heterocyclic γ‐amino acids built around a thiazole ring, named ATCs. ATC oligomers are helical molecules resulting from the formation of a highly stable C9 hydrogen‐bonding pattern. Following our program, we herein address the potential of ATC oligomers as tunable scaffolds for the development of original multivalent glycoclusters.