
Category: 2019


Chemical cross-linking methods for cell encapsulation in hydrogels

Chemical insights into bioinks for 3D printing

Self-mineralization and assembly of a bis-silylated Phe–Phe pseudodipeptide to a structured bioorganic–inorganic material
Organocatalytic Asymmetric Addition of Aldehyde to Nitroolefin by H-d-Pro-Pro-Glu-NH2: A Mechanistic Study
The HslV Protease from Leishmania major and Its Activation by C-terminal HslU Peptides
Structure and dynamics of G protein-coupled receptor-bound ghrelin reveal the critical role of the octanoyl chain

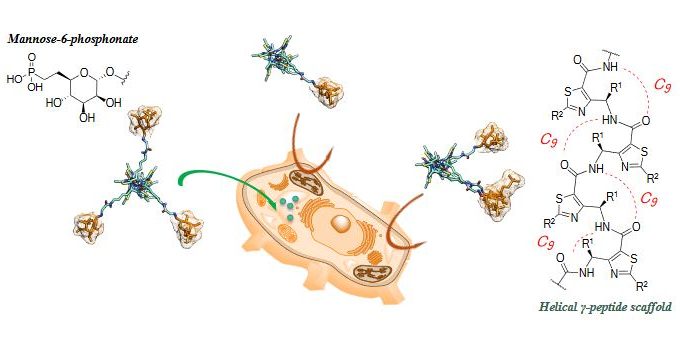
Topological Requirements for CI-M6PR-Mediated Cell Uptake
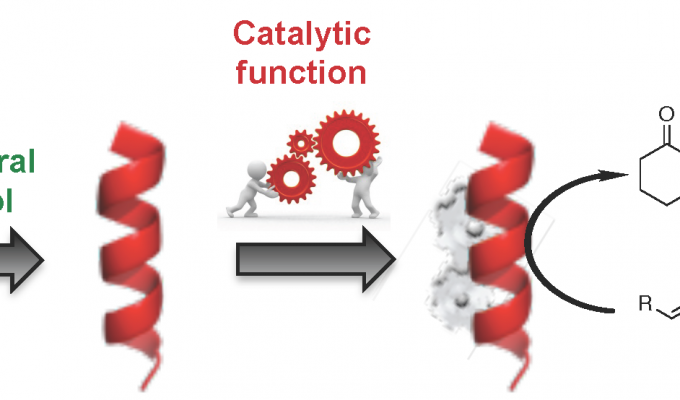
Prospect of Thiazole-based gamma-Peptides Foldamers in Enamine Catalysis: Exploration of the Nitro-Michael Addition


