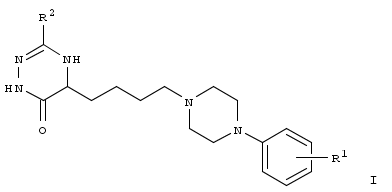
Abstract
A series of arylpiperazinylbutyl derivs. I [R1 = H, 2-OMe, 2-SMe, 2-F, 4-Cl, 2,3-diCl; R2 = Ph, 2-ClC6H4, cyclohexyl] of 4,5-dihydro-1,2,4-triazine-6(1H)-ones was designed and synthesized according to the new solid-supported methodol. In this approach, triazinone scaffold was constructed from the fmoc-protected glycine. The library representatives showed different levels of affinity for 5-HT7 and 5-HT1A receptors, among which compds. I [R1 = 2-SMe, 2-F; R2 = Ph and R1 = 2-OMe, 2-SMe, 2,3-diCl; R2 = cyclohexyl] were classified as dual 5-HT7/5-HT1A receptors ligands. The structure-affinity relationship anal. revealed that receptor affinity and selectivity of the tested compds. depended on the kind of substituent in position 3 of triazinone fragment as well as substitution pattern of phenylpiperazine moiety.