Abstract
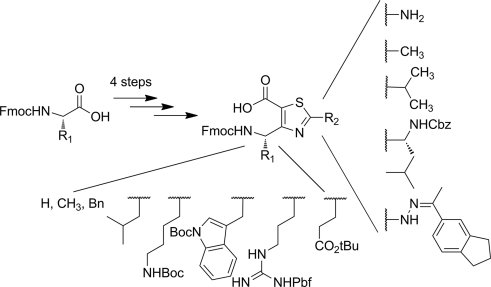
4-Amino(methyl)-1,3-thiazole-5-carboxylic acids (ATCs) are a new class of constrained heterocyclic γ-amino acids built around a thiazole ring; these compds. are valuable as design mimics of the secondary structures of proteins such as helixes, β-sheets, turns, and β-hairpins. We report herein a short and versatile chem. route to orthogonally protected ATCs. The synthesis is centered on cross-Claisen condensations between N-Fmoc-amino acids and sterically hindered 1,1-dimethylallyl acetate. The optimized conditions are compatible with aliph., arom., acidic, and basic amino acids. The resulting N-Fmoc-β-keto ester intermediates were engaged in a two-step process to give ATCs in 45-90 % yields. The synthetic protocol provides a highly flexible method for the introduction of a wide variety of lateral chains either on the γ-carbon atom or on the thiazole core of the γ-amino acids.