Author: Lubomir Vezenkov
Inhibitors of kallikrein-related peptidases: An overview.
Synthesis of [1,2,4]Triazolo[4,3- a]piperazin-6-ones: An Approach to the Triazole-Fused Ketopiperazine Scaffold
GHSR-D2R heteromerization modulates dopamine signaling through an effect on G protein conformation

Cecile Echalier, presents her PhD work in SCF video
ORALCANCERPRINT

Gilles Subra invited at E-MRS (18-22 June) in Strasbourg

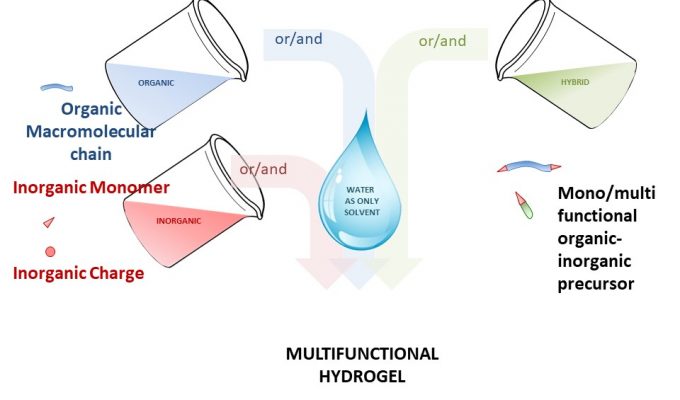
Laurine presented Legogel project at European Material Research Symposium in Strasbourg (France)