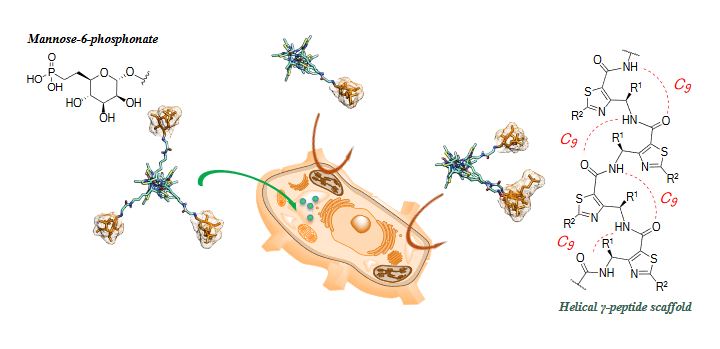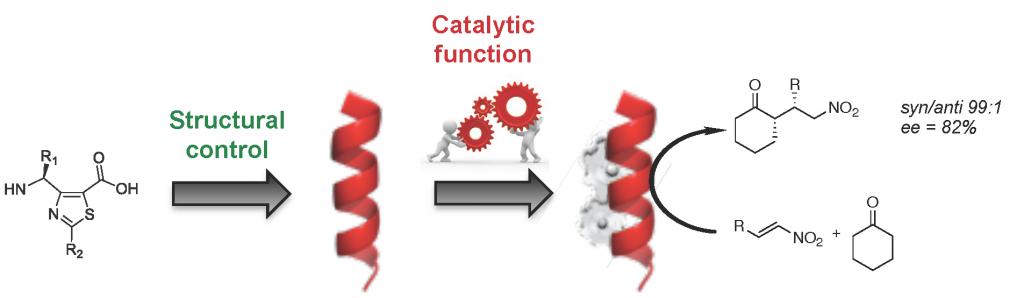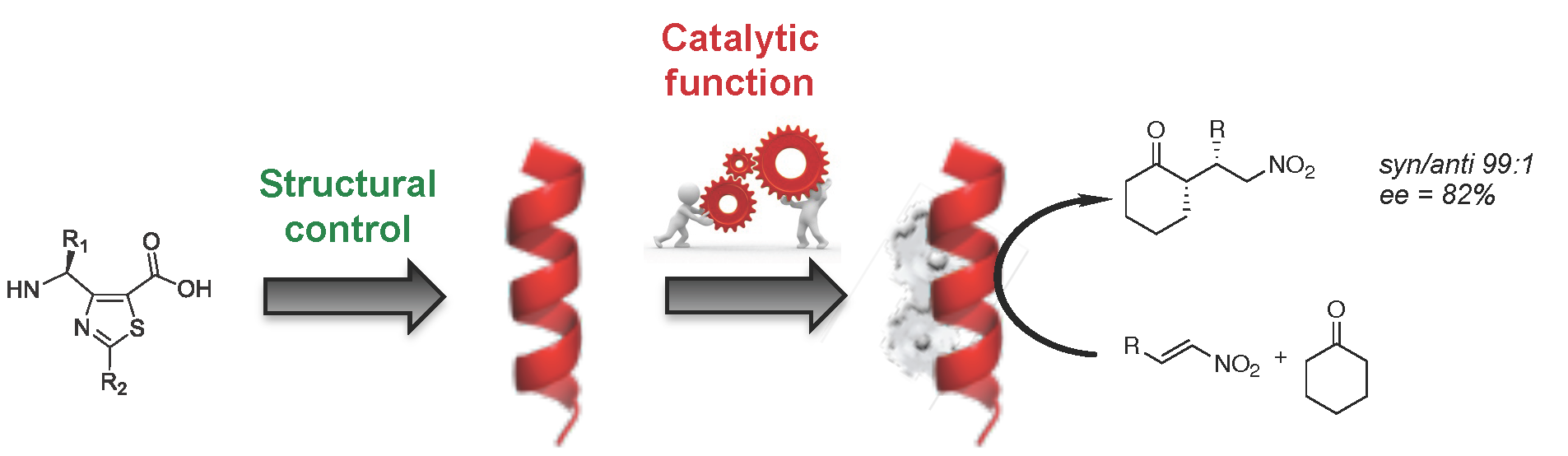
Our group synthesizes stapled peptides and pseudopeptide oligomers constructed from constrained β- and γ-amino acids or dipeptide mimetics able to adopt stable and predictable helical and ribbon conformations. Due to their well-defined secondary structure and their resistance to enzyme degradation, stapled peptides and foldamers are used for various biological applications, in particular for the development of efficient vectors for drug delivery, antimicrobials, inhibitors of protein-protein interactions and the targeting of the mannose 6-phosphate receptor for non-invasive cancer therapy. Bio-inspired foldamer catalysts built on heterocyclic γ-peptide scaffolds are also currently developed.
Synthesis and structural analysis of foldamers
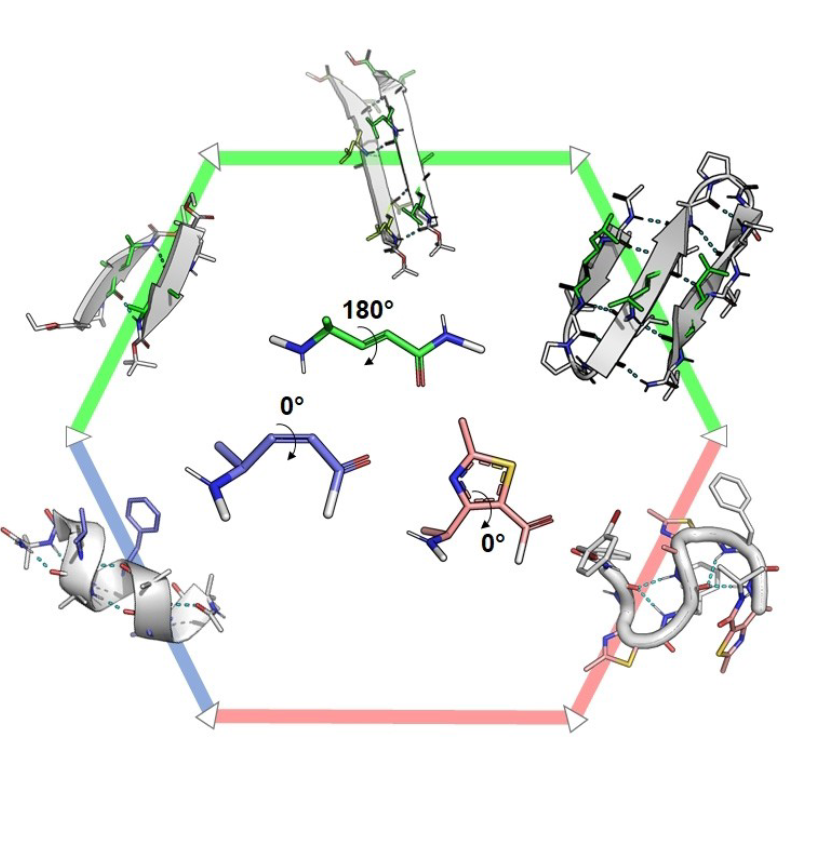
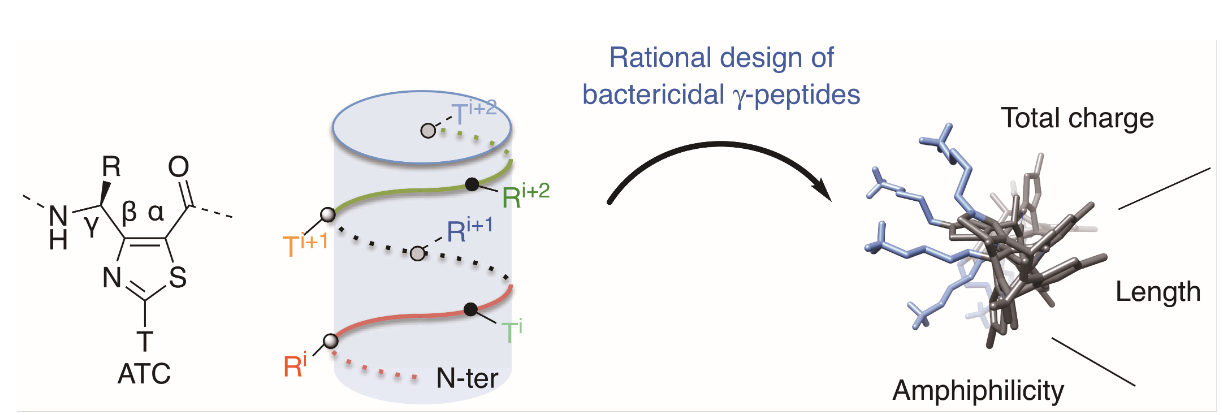
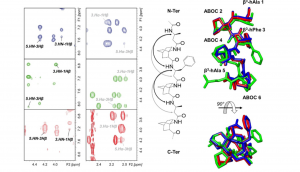
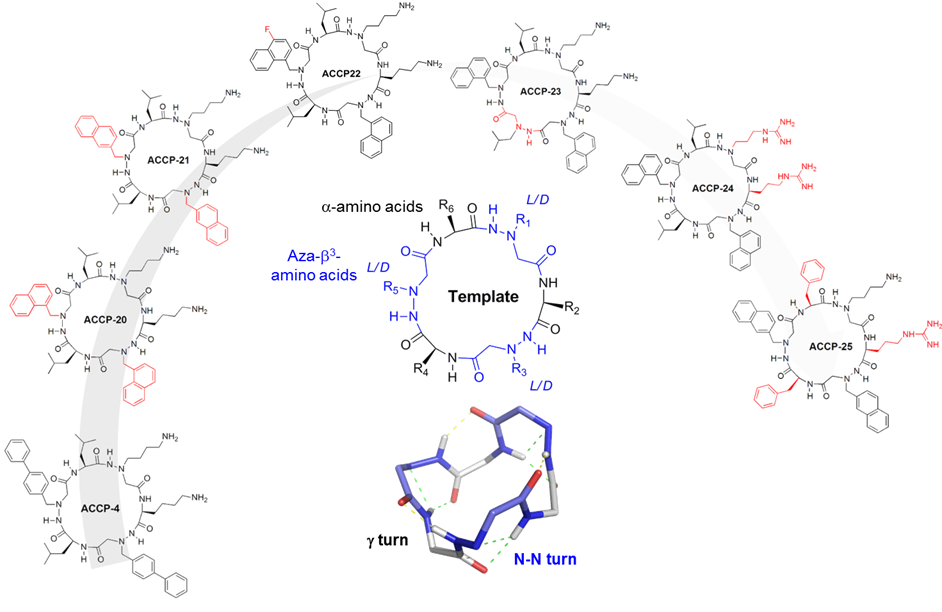
We synthesize and characterize various homo- and hetero-oligomers using the ABOC, ATC residues which are constrained β- and γ-building blocks respectively, and Agl-AA dipeptide mimics. Depending on the sequence, we obtained different architectures, from various helices to ribbons.
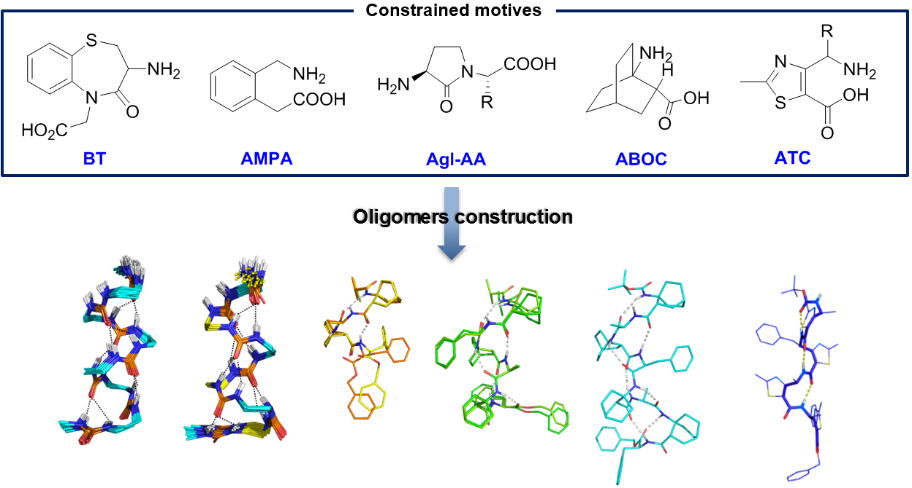
Numerous techniques, i.e. CD, FTIR and NMR spectroscopies, X-ray diffraction and DFT calculations are combined to characterize the three dimensional structure of the various oligomers.
Interaction with proteins
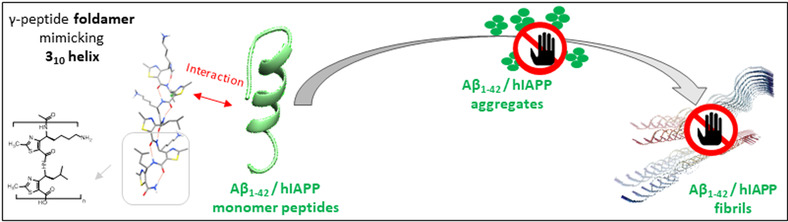
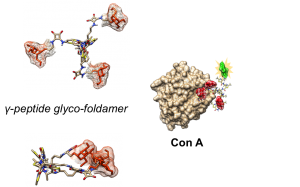
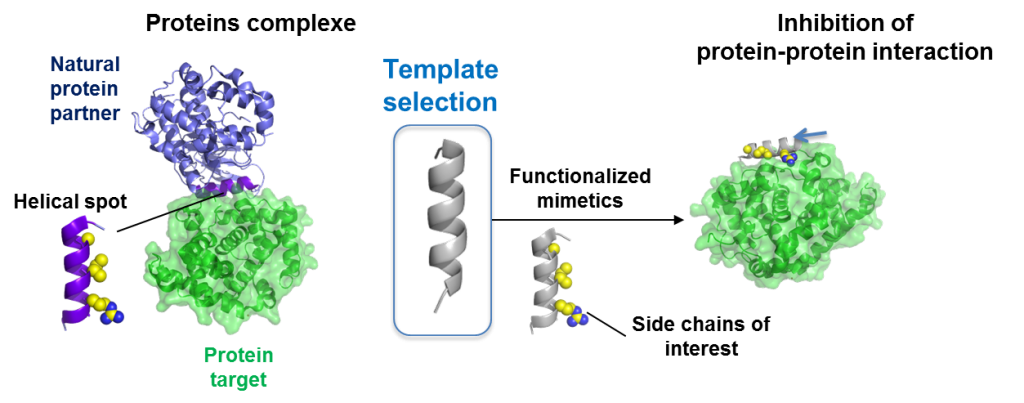
Stapled peptides and foldamers are currently evaluated for their ability to regulate physiological processes inhibiting protein-protein interactions, proteins aggregation in various pathological context such as cancers or Alzheimer disease as example. We also developed original glycofoldamers targeting the mannose 6-phosphate receptor overexpressed in prostate cancer cell lines and tissues.
Development of antimicrobial foldamers
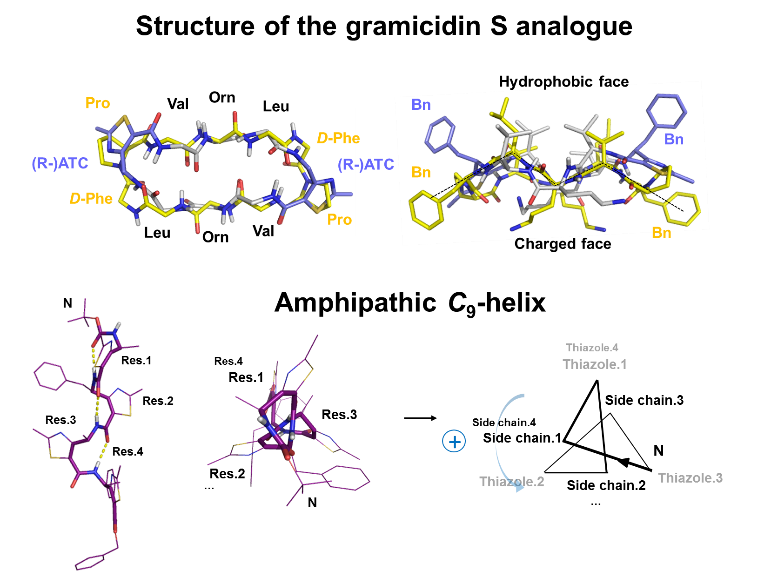
A bioactive analogue of gramicidin S was successfully designed using an ATC building block as a turn inducer. The NMR solution structure of the analogue adopted an antiparallel β-pleated sheet conformation similar to that of the natural compound. The hybrid α,γ-cyclopeptide exhibited significant reduced hemotoxicity compared to gramicidin S, while maintaining strong antibacterial activity [1-3]. New highly selective sequences exhibiting strong antimicrobial activities are currently developed.