MURIEL AMBLARD
Senior researcher, CNRS MONTPELLIER
Dr Muriel AMBLARD obtained her PhD in Organic Chemistry at the University of Montpellier under the supervision of Prof Jean Martinez in 1991. She joined the group of Dr Paul Anderson at Merck Sharp & Dohm in West point (Pennsylvania, USA) as a post-doctoral fellow. In 1996, she obtained a position at the Laboratory of Amino Acid Peptide and Protein in Montpellier as a CNRS researcher. She became a CNRS senior researcher and a team leader at the Institute of Biomolecules Max Mousseron in 2007. Her research interests are mainly at the interface of chemistry, biology and analysis. She developed a number of potent receptor agonists and antagonists of several peptide hormones. Related to medicinal chemistry programs, she developed new multifunctional scaffolds and focused on the design and synthesis of small molecules (diazepinones, spiroimidazolidinones, constraint dipeptide mimics) by combinatorial chemistry on solid support.
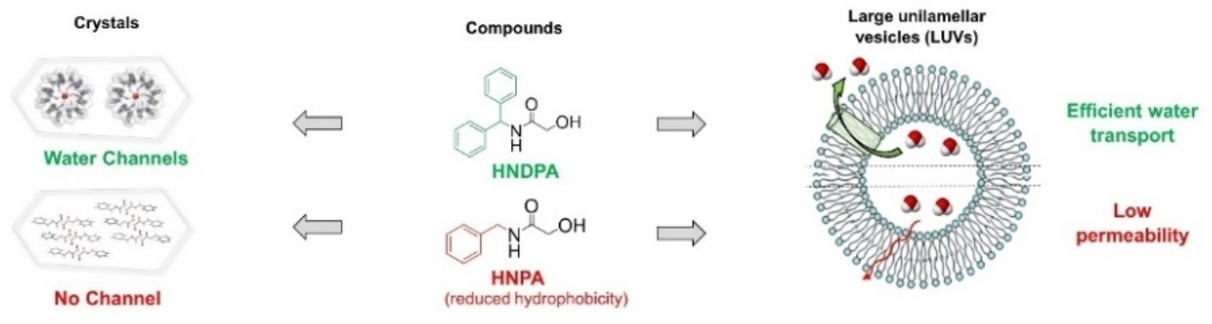
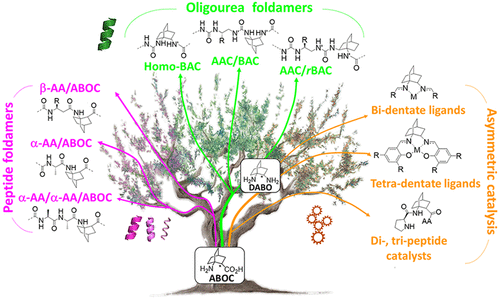
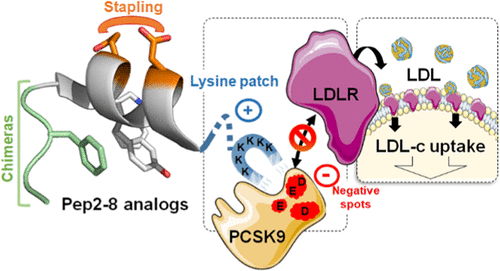
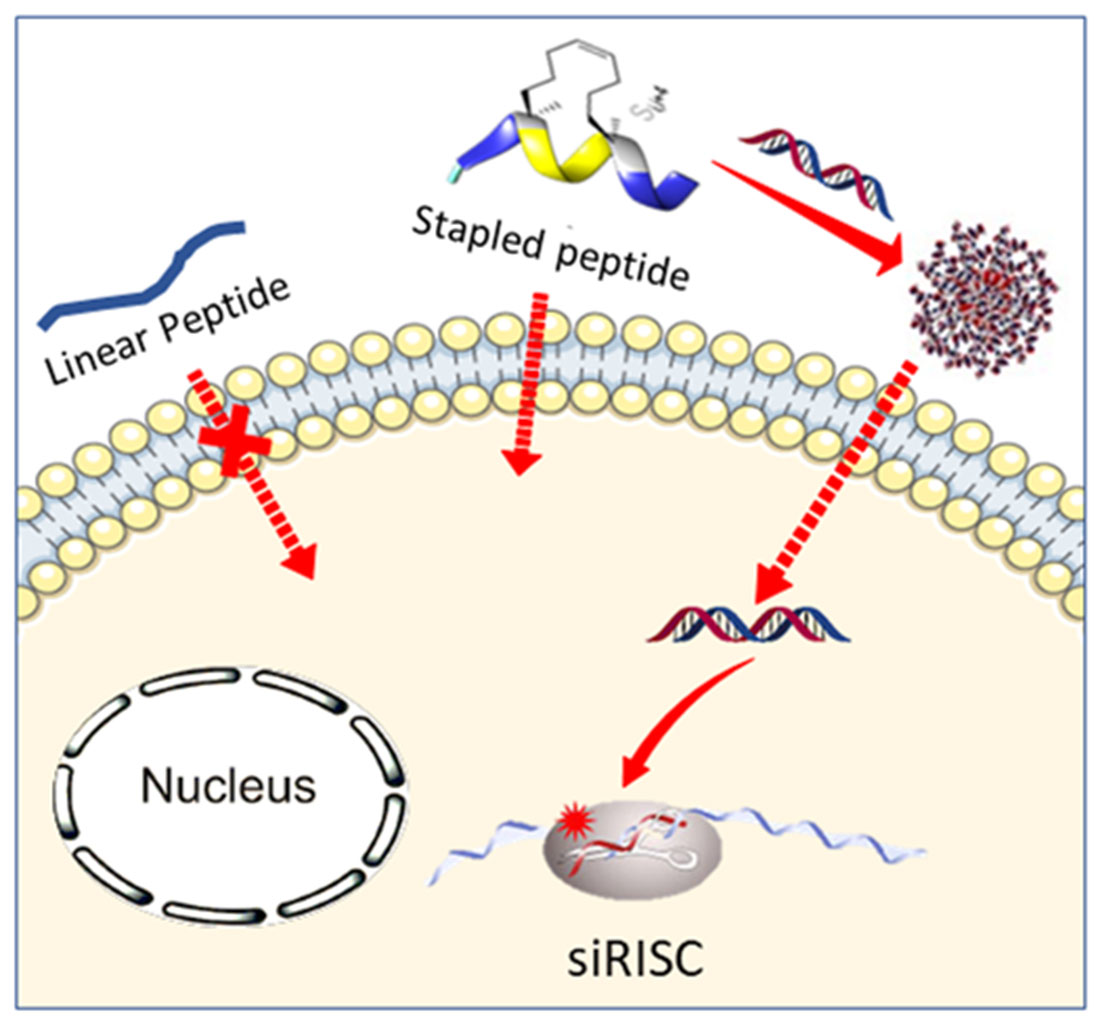
Her recent work focuses on the design and synthesis of constrained β-amino acid and dipeptide mimic oligomers as highly predictable and stable helical molecular architectures (Foldamers) applied to 1) the identification of cell penetrating compounds for the delivery of bioactive compounds inside the cells and 2) inhibitors of protein/protein interactions.
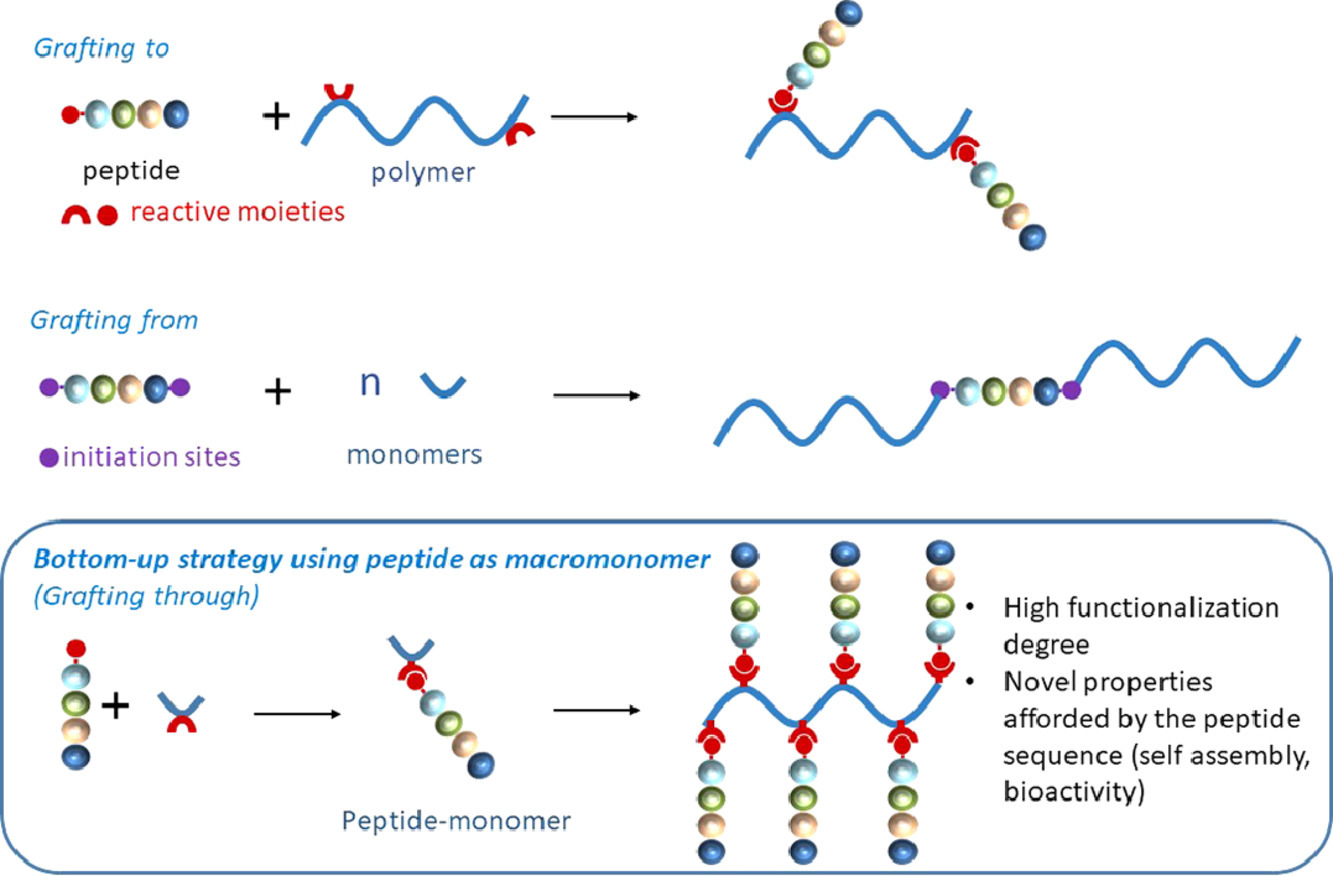
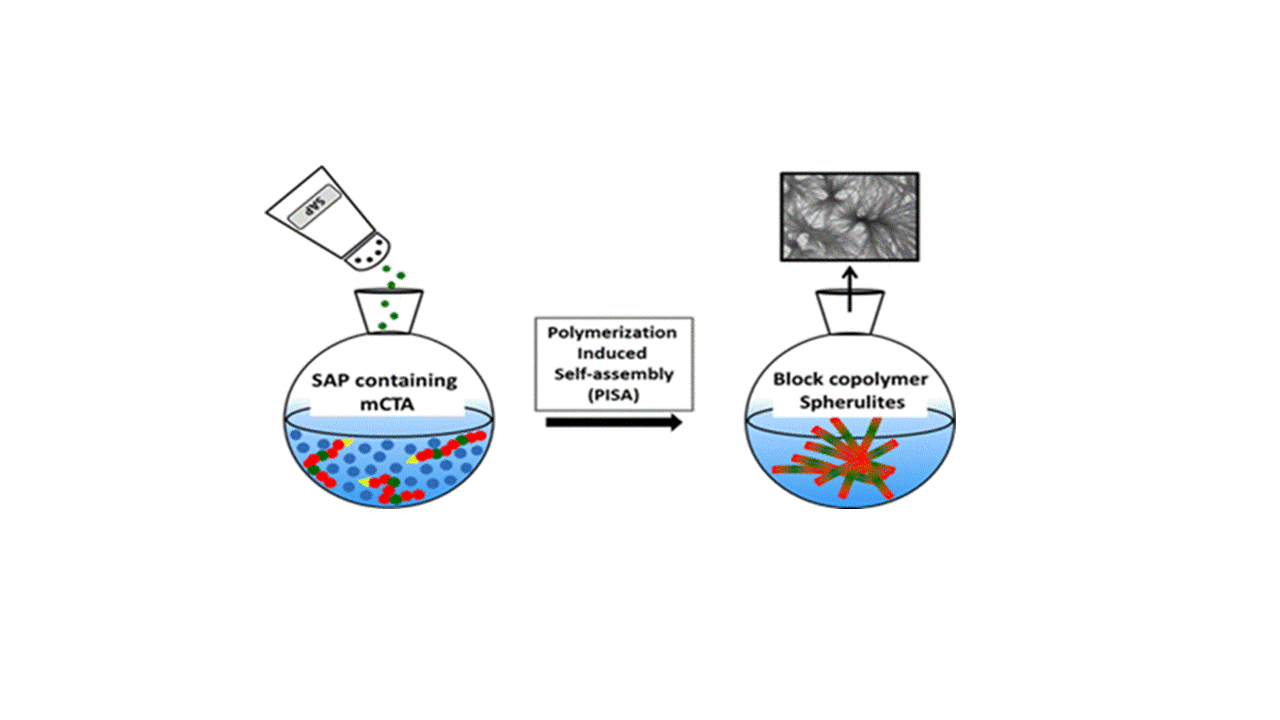
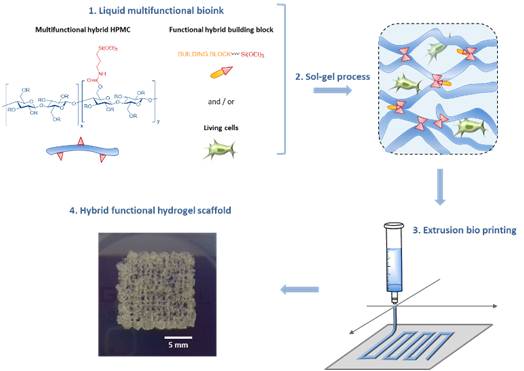
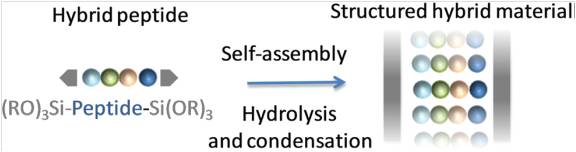
Another part of her research is devoted to the development of (1) new methodologies in peptide synthesis (the use of an O-N acyl transfer reaction for the synthesis of cyclic peptides without epimerization, new strategy for the synthesis of peptides alcohols on solid support, synthesis of stapled peptides ans peptide-polymers….) and (2) peptide-based biopolymers by new approaches relying on bottom up polymerisation of peptide-activated monomer.
Muriel is co-author of over 85 papers, 5 book chapters and 7 patents.
Contact:
muriel.amblard@umontpellier.fr
0033411759605
5 major publications :
D. Paramelle, G. Subra, L. Vezenkov, M. Maynadier, C. André, C. Enjalbal, M. Calmès, M. Garcia, J. Martinez, M. Amblard. A straightforward approach for cellular uptake quantification. Angew. Chem. Int. Ed. 2010, 49, 8240-8243.
B. Legrand, C. André, E. Wenger, C. Didierjean, M C. Averlant-Petit, J. Martinez, M. Calmes, M. Amblard.Robust Helix Formation in a New Family of oligoureas based on a Constrained Bicyclic Building Block, Angew. Chem. Int. Ed., 2012, 51, 11267-11270
B. Legrand, C. André, L. Moulat, E. Wenger, C. Didierjean, E. Aubert, M C. Averlant-Petit, J. Martinez, M. Calmes, M. Amblard.Unprecedented Chain-Length Dependent Conformational Conversion Between 11/9 and 18/16 Helix inα/β-Hybrid Peptides. Angew. Chem. Int. Ed., 2014, 53, 13131-13135.
A. Martin, B. Legrand, L. L. Vezenkov, M. Berthet, G. Subra, M. Calmès, J.L. Bantignies, J. Martinez, M. Amblard. Turning peptide sequences into ribbon foldamers via a straightforward multi-cyclization reaction. Angew. Chem. Int. Ed., 2015, 54, 13966 –13970
B. Legrand, C. André, L. Moula, C. Didierjean, P. Hermet, J.L. Bantignies, J. Martinez, M. Amblard, M. Calmès, 12/14/14-Helix Formation in 2:1 α/β-Hybrid Peptides Containing Bicyclo[2.2.2]octane Ring Constraints, Chemistry: a European Journal, 2016, 22, 11986 –11990