Chemistry. 2017 Dec 11;23(69):17584-17591. doi: 10.1002/chem.201704001. Epub 2017 Nov 15.
Bonnel C, Legrand B, Simon M, Martinez J, Bantignies JL, Kang YK, Wenger E, Hoh F, Masurier N, Maillard LT.
Abstract
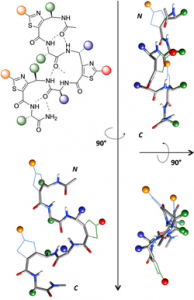
According to their restricted conformational freedom, heterocyclic γ-amino acids are usually considered to be related to Z-vinylogous γ-amino acids. In this context, oligomers alternating α-amino acids and thiazole-based γ-amino acids (ATCs) were expected to fold into a canonical 12-helical shape as described for α/γ-hybrid peptides composed of cis-α/β-unsaturated γ-amino acids. However, through a combination of X-ray crystallography, NMR spectroscopy, FTIR experiments, and DFT calculations, it was determined that the folding behavior of ATC-containing hybrid peptides is much more complex. The homochiral α/(S)-ATC sequences were unable to adopt a stable conformation, whereas the heterochiral α/(R)-ATC peptides displayed novel ribbon structures stabilized by unusual C9/12 -bifurcated hydrogen bonds. These ribbon structures could be considered as a succession of pre-organized γ/α dipeptides and may provide the basis for designing original α-helix mimics.