J. Med. Chem. 2020, 63, 17, 9168–9180. https://doi.org/10.1021/acs.jmedchem.0c00077
Abstract
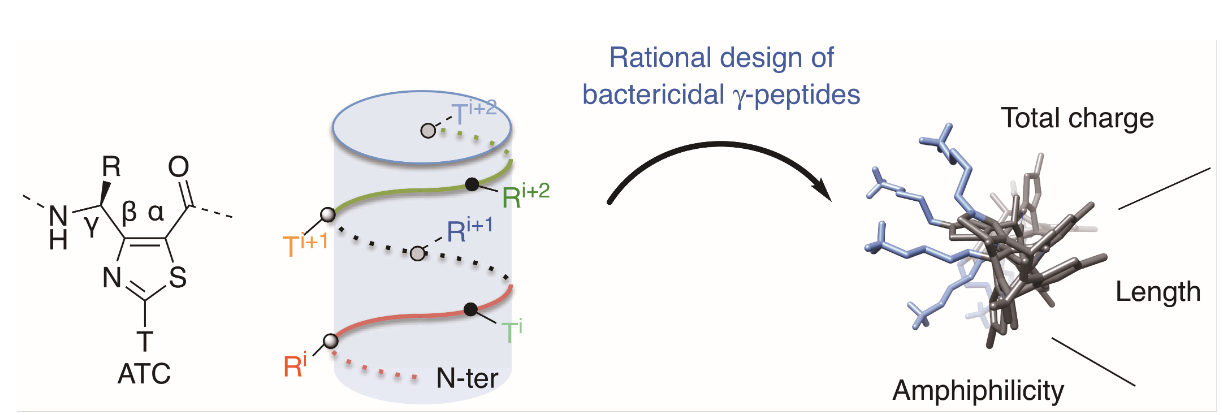
Antimicrobial peptides (AMPs) are amphipathic molecules displaying broad-spectrum bactericidal activity, providing opportunities to develop a new generation of antibiotics. However, their use is limited either by poor metabolic stability or by high hemolytic activity. We herein addressed the potential of thiazole-based γ-peptide oligomers named ATCs as tunable scaffolds to design polycationic AMP mimetics. Knowing the side chain distribution along the backbone, we rationally designed facially amphiphilic sequences with bactericidal effect in the micromolar range. Since no hemolytic activity was detected up to 100 μM, this class of compounds has shown the potential for therapeutic development.