Abstract
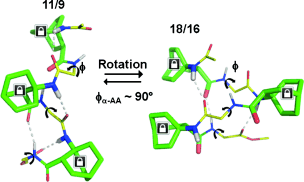
α,β-Hybrid oligomers of varying lengths with alternating proteogenic α-amino acid and the rigid β2,3,3-trisubstituted bicyclic amino acid ABOC residues were studied using both x-ray crystal and NMR soln. structures. While only an 11/9 helix was obtained in the solid state regardless of the length of the oligomers, conformational polymorphism as a chain-length-dependent phenomenon was obsd. in soln. Consistent with DFT calcns., short oligomers adopted an 11/9 helix, whereas an 18/16 helix was favored for longer oligomers in soln. A rapid interconversion between the 11/9 helix and the 18/16 helix occurred for oligomers of intermediate length.