Jean-françois hernandez
Research Director (CNRS)
Jean-François Hernandez performed his PhD in the laboratory of Prof. Bernard P. Roques (Paris V University, 1987) under the supervision of Prof. Marie-Claude Fournié-Zaluski, where he developed pseudo-dipeptidic inhibitors of enkephalin-degrading enzymes as potential analgesics. Then, he went to the Salk Institute in San Diego as a post-doctoral fellow (1988-1990) under the supervision of Prof. Jean E. Rivier, where he learned solid phase peptide synthesis (Boc and Fmoc chemistries, GRF agonists, CRF antagonists, conotoxins, long peptides).
After moving back to France, he obtained a two-years temporary assistant professor position (ATER, 1990-1992) in the laboratory of Prof. Joëlle Paris at the faculty of Pharmacy of Lyon, where he developed SPPS methodology and worked on a project related to immunology. He obtained a permanent research position at the CNRS in 1992 and joined the team of Gérard Arlaud at Institut de Biologie Structurale in Grenoble, where he mainly performed the solid phase synthesis of peptides for fundamental and/or structural studies. He also contributed to the characterization of natural peptidase inhibitors (squash trypsin inhibitors like MCoTI) and of microbial peptidases.
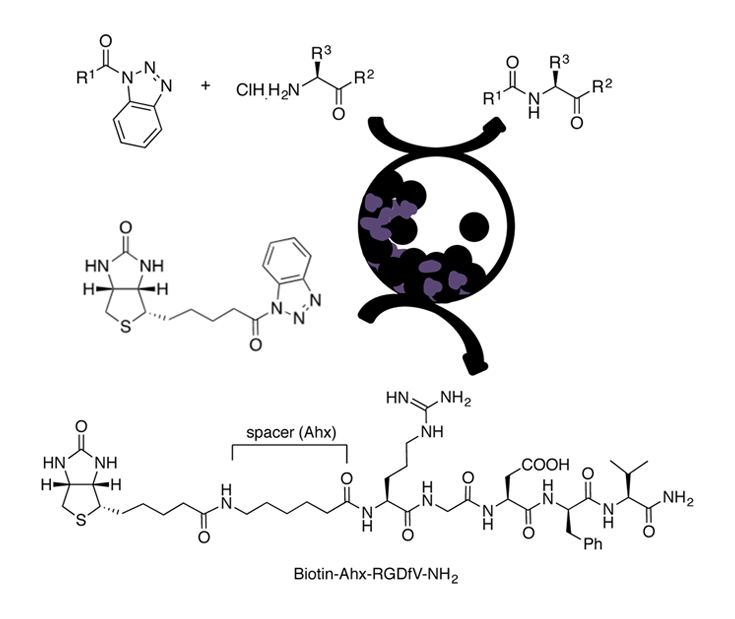
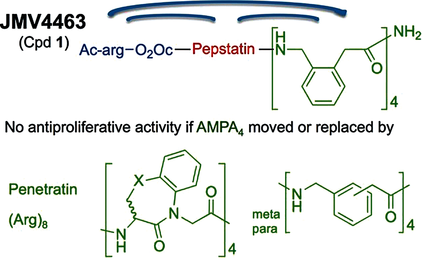
In 2001, he moved to the laboratory of Prof. Jean Martinez (Laboratoire des Amino acides, Peptides et Protéines) in Montpellier, where he started to supervise a small group devoted to the development of enzyme inhibitors (pseudo-peptides, heterocycles) with therapeutical interest. Among targets that were or are currently explored: parasitic peptidases (Plasmodium, Leishmania), beta-lactamases, NO Synthases, Cathepsin D, secretases. A second important part of its activity is the development of solid phase synthetic strategies in the peptide/pseudopeptide field. In particular, he developed several strategies for the preparation of arginine- and arginine-like-containing compounds.
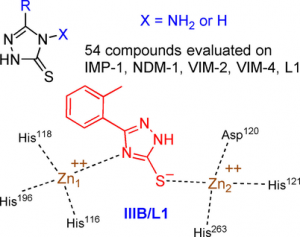
Currently, his activity mainly focuses on: (i) the development of heterocyclic molecules as inhibitors of metallo-β-lactamases, which are bacterial enzymes responsible of their resistance to carbapenem antibiotics, a major threat to human health; (ii) the development of pseudo-peptides inhibiting a peptidase of the Malaria-causing parasites Plasmodium falciparum and vivax.
Contact:
jean-francois.hernandez@umontpellier.fr
+33 (0)4 48 79 21 84
5 major publications :
Verdirosa F., Gavara L., Sevaille L., Tassone G., Corsica G., Legru A., Feller G., Chelini G., Mercuri P. S., Tanfoni S., Sannio F., Benvenuti M., Cerboni G., De Luca F., Bouajila E., Vo Hoang Y., Licznar-Fajardo P., Galleni M., Pozzi C., Mangani S., Docquier J.-D. & Hernandez J.-F. (2022) 1,2,4-Triazole-3-thione analogues with a 2-ethylbenzoic acid at position 4 as VIM-type metallo-β-lactamase inhibitors. ChemMedChem 17, e202100699.
Singh P., Samanta K., Kebe N. M., Michel G., Legrand B., Sitnikova V. E., Kajava A., Pagès M., Bastien P., Pomares C., Coux O. & Hernandez J.-F. (2022) The C-terminal segment of Leishmania major HslU: toward potential inhibitors of HslVU activity. Bioorg. Chem. 119, 105539.
Touati-Jallabe Y., Tintillier T., Mauchauffée E., Boucher J.-L., Leroy J., Ramassamy B., Hamzé A., Mezghenna K., Bouzekrini A., Verna C., Martinez J., Lajoix A.-D. & Hernandez J.-F. (2020) Solid phase synthesis of substrate-based dipeptides and heterocyclic pseudo-dipeptides as potential NO Synthase inhibitors. ChemMedChem 15, 517-531.
Gavara L., Sevaille L., De Luca F., Mercuri P., Bebrone C., Feller G., Legru A., Cerboni G., Tanfoni S., Baud D., Cutolo G., Bestgen B., Chelini G., Verdirosa F., Sannio F., Pozzi C., Benvenuti M., Kwapien K., Fischer M., Becker K., Frère J.-M., Mangani S., Gresh N., Berthomieu D., Galleni M., Docquier J.-D. & Hernandez J.-F. (2020) 4-Amino-1,2,4-triazole-3-thione-derived Schiff bases as metallo-β-lactamase inhibitors. Eur. J. Med. Chem. 208:112720.
Sevaille L., Gavara L., Bebrone C., De Luca F., Nauton L., Achard M., Mercuri P., Tanfoni S., Borgianni L., Guyon C., Lonjon P., Turan-Zitouni G., Dzieciolowski J., Becker K., Bénard L., Condon C., Maillard L., Martinez J., Frère J.-M., Dideberg O., Galleni M., Docquier J.-D. & Hernandez J.-F. (2017) 1,2,4-Triazole-3-thione compounds as inhibitors of di-zinc metallo-β-lactamases. ChemMedChem 12, 972-985.