Abstract
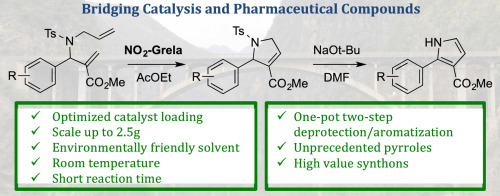
Ring-closing metathesis (RCM) is a powerful tool for the prepn. of cyclic org. compds. Yet, one of the major limitations of this method is the difficulty to prep. large quantities of target mols. Herein we describe a comprehensive study regarding the gram-scale synthesis of 2-aryl-1H-pyrrole-3-carboxylates based on the ring-closing metathesis of the corresponding β-amino esters as a key step. This study includes evaluation of solvent and catalyst as well as reaction kinetics on the RCM. After an aromatization step, this methodol. allowed for an efficient generation of variously substituted and unprecedented 2-aryl-1H-pyrrole-3-carboxylates in good yields and cost-effectiveness. The resulting mols. might serve as key building blocks for the generation of CNS-oriented compd. libraries.