
Baptiste legrand
Research Engineer, University of Montpellier (UM)
Baptiste received his PhD in life sciences and health (structural biology) from the University of Rennes 1 in 2009 and moved for a two-years post-doctoral fellowship at the Laboratory of Macromolecular Chemistry and Physics (LCPM, Nancy) working on the characterization of foldamers structures combining various techniques (NMR, CD and FTIR spectroscopies, and Molecular Dynamics).
He joined the IBMM in 2012. His research is devoted to the development of rationally designed architectures in the field of health and catalysis. He is interested in the determination of three-dimensional structures, deciphering the mechanisms that govern the folding and stability of complex edifices at the atomic scale, and peptide self-assemblies. The main applications concern the development of antimicrobials and protein-protein interaction inhibitors.
Contact:
baptiste.legrand@umontpellier.fr
+33 448792185
5 major publications :
Legrand B, André C, Moulat L, Didierjean C, Hermet P, Bantignies JL, Martinez J, Amblard M, Calmès M. 12/14/14-Helix Formation in 2:1 α/β-Hybrid Peptides Containing Bicyclo[2.2.2]octane Ring Constraints. Chemistry. 2016. 16;22(34):11986-90.
Martin V, Legrand B, Vezenkov LL, Subra G, Calmès M, Bantignies J.L, Martinez J, Amblard M. Turning Peptide Sequences into Ribbon Foldamers by a Straightforward Multicyclization Reaction. Angew. Chem. Int. Ed. 2015. 16;54(47):13966-70.
Legrand B, André C, Moulat L, Wenger E, Didierjean C, Aubert E, Averlant-Petit MC, Martinez J, Calmes M, Amblard M. Unprecedented chain-length-dependent conformational conversion between 11/9 and 18/16 helix in α/β-hybrid peptides. Angew Chem Int Ed. 2014. 53(48):13131-5.
Mathieu L, Legrand B, Deng C, Vezenkov L, Wenger E, Didierjean C, Amblard M, Averlant-Petit MC, Masurier N, Lisowski V, Martinez J, Maillard LT. Helical oligomers of thiazole-based γ-amino acids: synthesis and structural studies. Angew Chem Int Ed. 2013. 3;52(23):6006-10.
Legrand B, André C, Wenger E, Didierjean C, Averlant-Petit MC, Martinez J, Calmes M, Amblard M. Robust helix formation in a new family of oligoureas based on a constrained bicyclic building block. Angew Chem Int Ed. 2012. 51(45):11267-70.
The Unexpected Helical Supramolecular Assembly of a Simple Achiral Acetamide Tecton Generates Selective Water Channels
Chemistry. 2022 Jun 10;28(33):e202200383. doi: 10.1002/chem.202200383
Dumitrescu DG, Rull-Barull J, Martin AR, Masquelez N, Polentarutti M, Heroux A, Demitri N, Bais G, Moraru IT, Poteau R, Amblard M, Krajnc A, Mali G, Legrand YM, van der Lee A, Legrand B
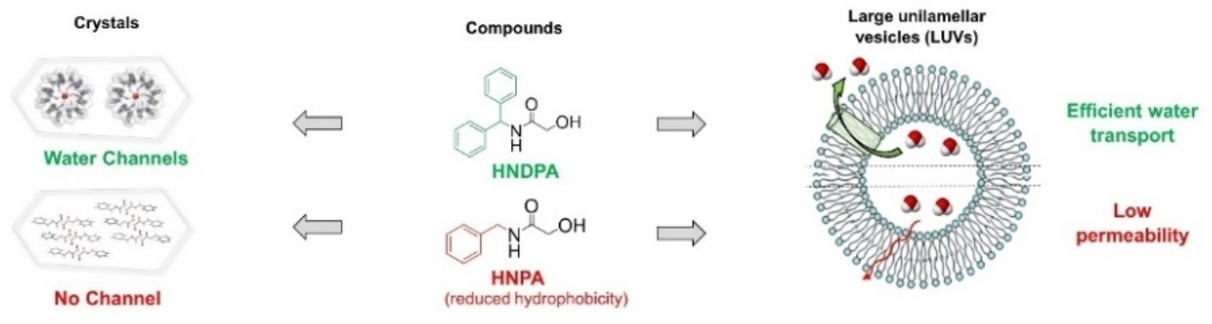
Abstract
Achiral 2-hydroxy-N-(diphenylmethyl)acetamide (HNDPA) crystallizes in the P61 chiral space group as a hydrate, building up permeable chiral crystalline helical water channels. The crystallization-driven chiral self-resolution process is highly robust, with the same air-stable crystalline form readily obtained under a variety of conditions. Interestingly, the HNDPA supramolecular helix inner pore is filled by a helical water wire. The whole edifice is mainly stabilized by robust hydrogen bonds involving the HNDPA amide bonds and CH… π interactions between the HNDPA phenyl groups. The crystalline structure shows breathing behavior, with completely reversible release and re-uptake of water inside the chiral channel under ambient conditions. Importantly, the HNDPA channel is able to transport water very efficiently and selectively under biomimetic conditions. With a permeability per channel of 3.3 million water molecules per second in large unilamellar vesicles (LUV) and total selectivity against NaCl, the HNDPA channel is a very promising functional nanomaterial for future applications.
α,β-Unsaturated γ-Peptide Foldamers
Chempluschem. 2021 Apr 1;86(4):629-645. doi: 10.1002/cplu.202100045. Online ahead of print.
Legrand B, Maillard LT
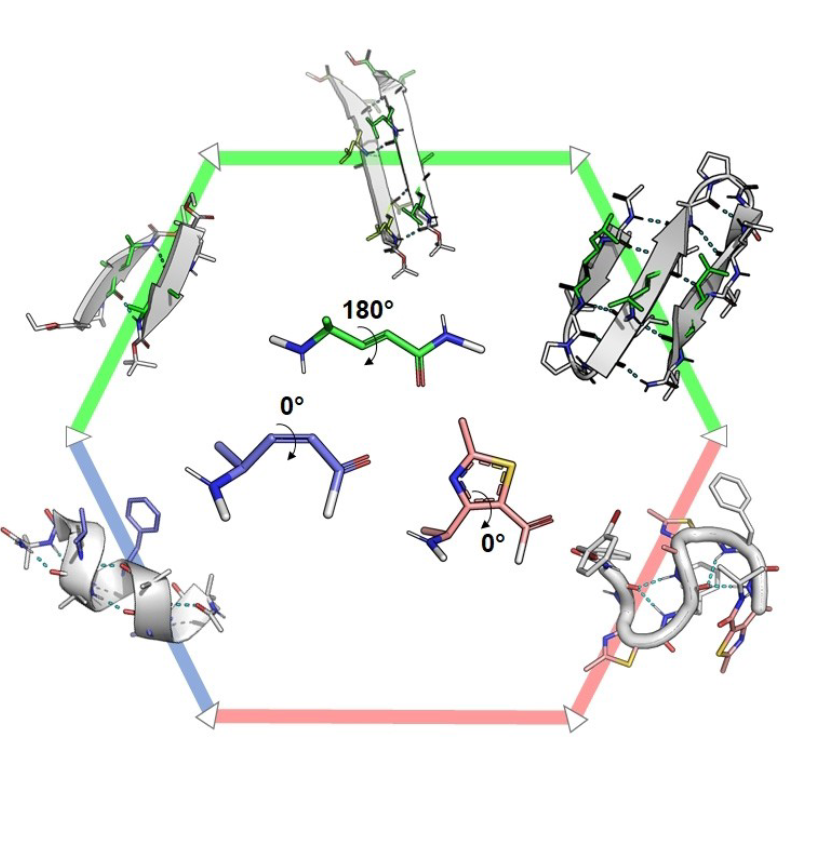
Abstract
Despite their concomitant emergence in the 1990s, γ-peptide foldamers have not developed as fast as β-peptide foldamers and to date, only a few γ-oligomer structures have been reported, and with sparse applications. Among these examples, sequences containing α,β-unsaturated γ-amino acids have recently drawn attention since the Z/E configurations of the double bond provide opposite planar restrictions leading to divergent conformational behaviors, from helix to extended structures. In this Review, we give a comprehensive overview of the developments of γ-peptide foldamers containing α,β-unsaturated γ-amino acids with examples of applications for health and catalysis, as well as materials science.
1-Aminobicyclo[2.2.2]octane-2-carboxylic Acid and Derivatives As Chiral Constrained Bridged Scaffolds for Foldamers and Chiral Catalysts
Acc Chem Res. 2021 Feb 2;54(3):685-696. doi: 10.1021/acs.accounts.0c00680. Epub 2021 Jan 19.
Milbeo P, Martinez J, Amblard M, Calmès M, Legrand B
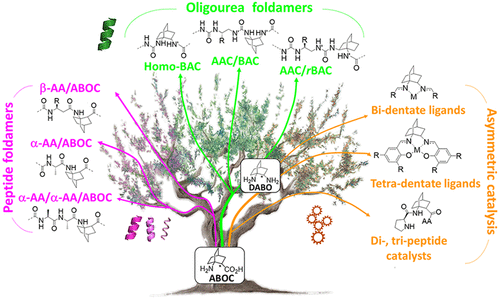
Abstract
The improvement of molecular diversity is one of the major concerns of chemists since the continuous development of original synthetic molecules provides unique scaffolds usable in organic and bioorganic chemistry. The challenge is to develop versatile platforms with highly controlled chemical three-dimensional space thanks to controlled chirality and conformational restraints. In this respect, cyclic β-amino acids are of great interest with applications in various fields of chemistry. In addition to their intrinsic biological properties, they are important precursors for the synthesis of new generations of bioactive compounds such as antibiotics, enzyme inhibitors, and antitumor agents. They have also been involved in asymmetric synthesis as efficient organo-catalysts in their free form and as derivatives. Finally, constrained cyclic β-amino acids have been incorporated into oligomers to successfully stabilize original structures in foldamer science with recent successes in health, material science, and catalysis. Over the last ∼10 years, we focused on bicyclic β-amino acids possessing a bicyclo[2.2.2]octane structure. This latter is a structural key element in numerous families of biologically active natural and synthetic products and is an interesting template for asymmetric synthesis. Nonetheless, reported studies on bicyclic carbo-bridged compounds are rather limited compared to those on bicyclic-fused and heterobridged derivatives. In this Account, we particularly focused on the synthesis and applications of the 1-aminobicyclo[2.2.2]octane-2-carboxylic acid, named, ABOC, and its derivatives. This highly constrained bicyclic β-amino acid, with a sterically hindered bridgehead primary amine and an endocyclic chiral center, displays drastically reduced conformational freedom. In addition, its high bulkiness strongly impacts the spatial orientation of the appended functionalities and the conformation of adjacent building blocks. Thus, we have first expanded a fundamental synthetic work by a wide ranging study in the field of foldamers, in the design of various stable peptide/peptidomimetic helical structures incorporating the ABOC residue (11/9-, 18/16-, 12/14/14-, and 12/10-helices). In addition, such bicyclic residue was fully compatible with and stabilized the canonical oligourea helix, whereas very few cyclic β-amino acids have been incorporated into oligoureas. In addition, we have pursued with the synthesis of some ABOC derivatives, in particular the 1,2-diaminobicyclo[2.2.2]octane chiral diamine, named DABO, and its investigation in chiral catalytic systems. Covalent organo-catalysis of the aldol reaction using ABOC-containing tripeptide catalysts provided a range of aldol products with high enantioselectivity. Moreover, the double reductive condensation of DABO with various aldehydes allowed the building of new chiral ligands that proved their efficiency in the copper-catalyzed asymmetric Henry reaction.
Potent Lys Patch-Containing Stapled Peptides Targeting PCSK9
J Med Chem. 2021 Aug 12;64(15):10834-10848. doi: 10.1021/acs.jmedchem.0c02051. Epub 2021 Jul 15.
Bourbiaux K, Legrand B, Verdié P, Mallart S, Manette G, Minoletti C, Stepp JD, Prigent P, Le Bail JC
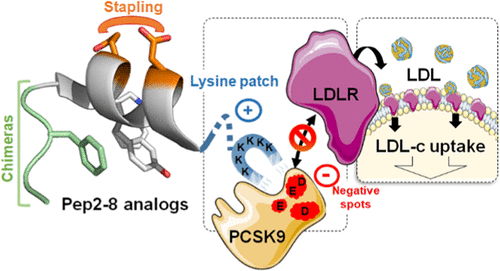
Abstract
Proprotein convertase subtilisin/kexin type 9 (PCSK9), identified as a regulator of low-density lipoprotein receptor (LDLR), plays a major role in cardiovascular diseases (CVD). Recently, Pep2-8, a small peptide with discrete three-dimensional structure, was found to inhibit the PCSK9/LDLR interaction. In this paper, we describe the modification of this peptide using stapled peptide and SIP technologies. Their combination yielded potent compounds such as 18 that potently inhibited the binding of PCSK9 to LDLR (KD = 6 ± 1 nM) and restored in vitro LDL uptake by HepG2 cells in the presence of PCSK9 (EC50 = 175 ± 40 nM). The three-dimensional structures of key peptides were extensively studied by circular dichroism and nuclear magnetic resonance, and molecular dynamics simulations allowed us to compare their binding mode to tentatively rationalize structure-activity relationships (SAR).
Hydrocarbon-Stapled Peptide Based-Nanoparticles for siRNA Delivery.
Nanomaterials (Basel). 2020 Nov 25;10(12):2334. doi: 10.3390/nano10122334
Simon M, Laroui N, Heyraud M, Laconde G, Ali LMA, Bourbiaux K, Subra G, Vezenkov LL, Legrand B, Amblard M, Bettache N.
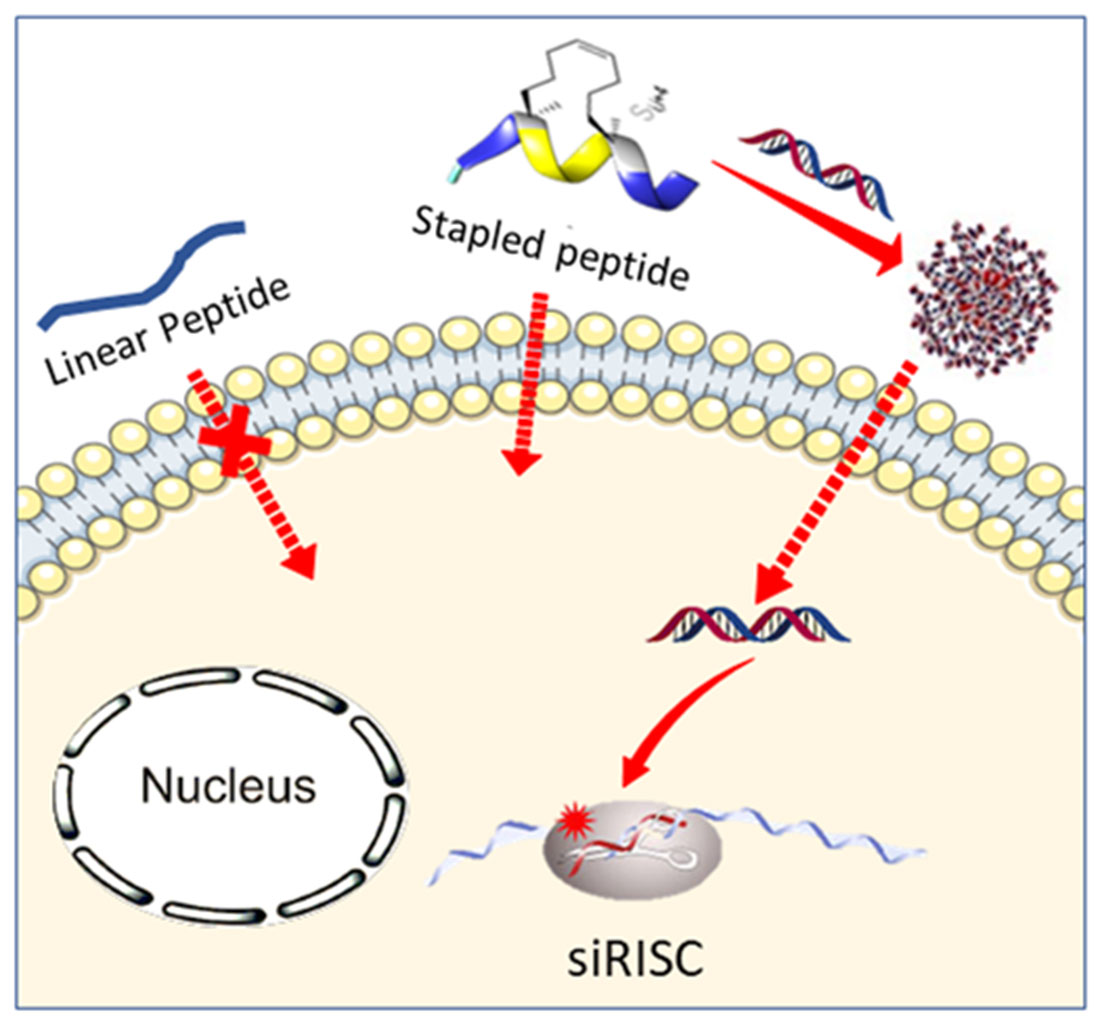
Abstract
Small interfering RNAs (siRNAs) are promising molecules for developing new therapies based on gene silencing; however, their delivery into cells remains an issue. In this study, we took advantage of stapled peptide technology that has emerged as a valuable strategy to render natural peptides more structured, resistant to protease degradation and more bioavailable, to develop short carriers for siRNA delivery. From the pool of stapled peptides that we have designed and synthesized, we identified non-toxic vectors that were able to efficiently encapsulate siRNA, transport them into the cell and induce gene silencing. Remarkably, the most efficient stapled peptide (JMV6582), is composed of only eight amino-acids and contains only two cationic charges.
Tailoring the Physicochemical Properties of Antimicrobial Peptides onto a Thiazole-Based γ-Peptide Foldamer
J. Med. Chem. 2020, 63, 17, 9168–9180. https://doi.org/10.1021/acs.jmedchem.0c00077
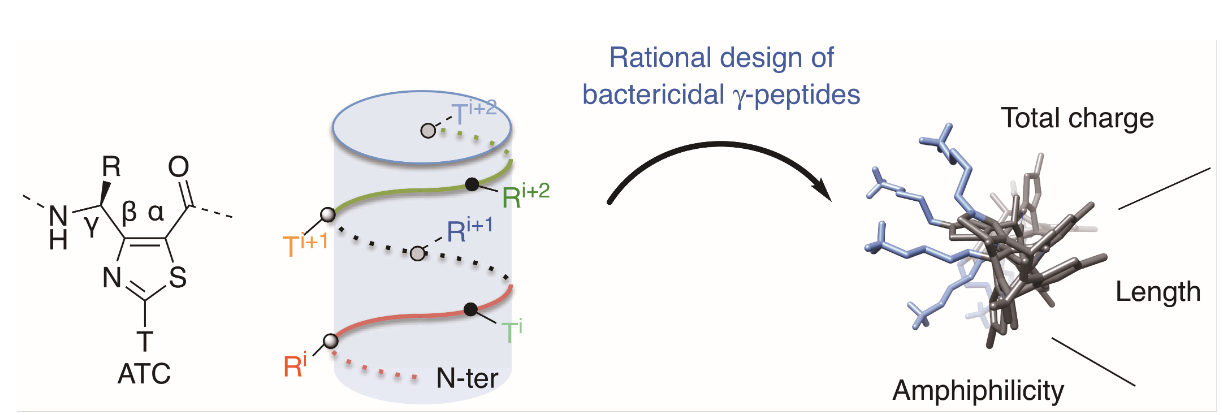
Abstract
Antimicrobial peptides (AMPs) are amphipathic molecules displaying broad-spectrum bactericidal activity, providing opportunities to develop a new generation of antibiotics. However, their use is limited either by poor metabolic stability or by high hemolytic activity. We herein addressed the potential of thiazole-based γ-peptide oligomers named ATCs as tunable scaffolds to design polycationic AMP mimetics. Knowing the side chain distribution along the backbone, we rationally designed facially amphiphilic sequences with bactericidal effect in the micromolar range. Since no hemolytic activity was detected up to 100 μM, this class of compounds has shown the potential for therapeutic development.
Helical γ-peptide foldamers as dual inhibitors of amyloid-β peptide and islet amyloid polypeptide oligomerization and fibrillization
Chem. Eur. J. 26, 14612-14622, https://doi.org/10.1002/chem.202001716
J. Kaffy J., Berardet C., Mathieu L., Legrand B., Taverna M., Halgand F., Van Der Rest G., Maillard L. T., Ongeri S.
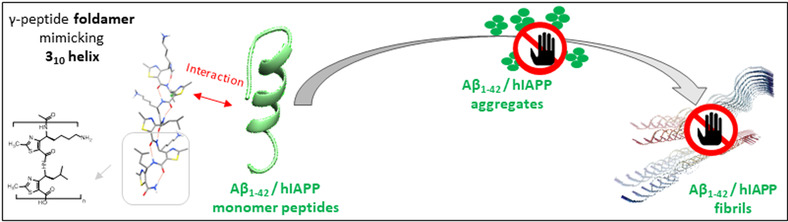
Abstract
Type 2 diabetes (T2D) and Alzheimer’s disease (AD) belong to the 10 deadliest diseases and are sorely lacking in effective treatments. Both pathologies are part of the degenerative disorders named amyloidoses, which involve the misfolding and the aggregation of amyloid peptides, hIAPP for T2D and Aβ1‐42 for AD. While hIAPP and Aβ1‐42 inhibitors have been essentially designed to target β‐sheet‐rich structures composing the toxic amyloid oligomers and fibrils of these peptides, the strategy aiming at trapping the non‐toxic monomers in their helical native conformation has been rarely explored. We report herein the first example of helical foldamers as dual inhibitors of hIAPP and Aβ1‐42 aggregation and able to preserve the monomeric species of both amyloid peptides. A foldamer composed of 4‐amino(methyl)‐1,3‐thiazole‐5‐carboxylic acid (ATC) units, adopting a 9‐helix structure reminiscent of 310 helix, was remarkable as demonstrated by biophysical assays combining thioflavin‐T fluorescence, transmission electronic microscopy, capillary electrophoresis and mass spectrometry.
Catalytic Foldamers: When the Structure Guides the Function
Abstract
Enzymes are predominantly proteins able to effectively and selectively catalyze highly complex biochemical reactions in mild reaction conditions. Nevertheless, they are limited to the arsenal of reactions that have emerged during natural evolution in compliance with their intrinsic nature, three-dimensional structures and dynamics. They optimally work in physiological conditions for a limited range of reactions, and thus exhibit a low tolerance for solvent and temperature conditions. The de novo design of synthetic highly stable enzymes able to catalyze a broad range of chemical reactions in variable conditions is a great challenge, which requires the development of programmable and finely tunable artificial tools. Interestingly, over the last two decades, chemists developed protein secondary structure mimics to achieve some desirable features of proteins, which are able to interfere with the biological processes. Such non-natural oligomers, so called foldamers, can adopt highly stable and predictable architectures and have extensively demonstrated their attractiveness for widespread applications in fields from biomedical to material science. Foldamer science was more recently considered to provide original solutions to the de novo design of artificial enzymes. This review covers recent developments related to peptidomimetic foldamers with catalytic properties and the principles that have guided their design.
Self-mineralization and assembly of a bis-silylated Phe–Phe pseudodipeptide to a structured bioorganic–inorganic material
Mater. Horiz., 2019, 6, 2040-2046 doi: 10.1039/C9MH00580C
Jebors S, Valot L, Echalier C, Legrand L, Mikhaleff R,Van Der Lee A, Arenal R, Dumy P, Amblard M, Martinez J, Mehdi A and Subra G.
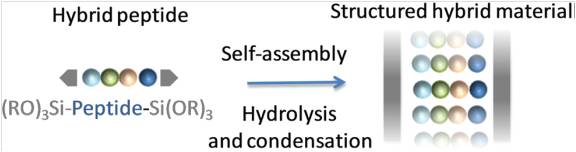
Abstract
Self-mineralization of a trialkoxysilyl hybrid peptide yields in a single step a nanostructured hybrid material. A bis-silylated pseudodipeptide inspired from the Phe–Phe dipeptide was used to program the assembly by sol–gel polymerization under heterogeneous conditions, in water at pH 1.5 without any structure-directing agent. A mechanism deciphering the hybrid material assembly was proposed thanks to 1H NMR spectroscopy. First, water-insoluble hybrid building blocks were hydrolysed into their soluble silanol counterparts. Then, these transitional species, thanks to hydrogen bonding and π–π stacking, self-assembled in solution. Last, the proximity of the silanol moieties favoured their polycondensation into growing siloxane oligomers, which spontaneously precipitated to produce an ordered hybrid material.
The HslV Protease from Leishmania major and Its Activation by C-terminal HslU Peptides
Int J Mol Sci. 2019 Feb 26;20(5). pii: E1021. doi: 10.3390/ijms20051021
Kebe NM, Samanta K, Singh P, Lai-Kee-Him J, Apicella V, Payrot N, Lauraire N, Legrand B, Lisowski V, Mbang-Benet DE, Pages M, Bastien P, Kajava AV, Bron P, Hernandez JF, Coux O
Abstract
HslVU is an ATP-dependent proteolytic complex present in certain bacteria and in the mitochondrion of some primordial eukaryotes, including deadly parasites such as Leishmania. It is formed by the dodecameric protease HslV and the hexameric ATPase HslU, which binds via the C-terminal end of its subunits to HslV and activates it by a yet unclear allosteric mechanism. We undertook the characterization of HslV from Leishmania major (LmHslV), a trypanosomatid that expresses two isoforms for HslU, LmHslU1 and LmHslU2. Using a novel and sensitive peptide substrate, we found that LmHslV can be activated by peptides derived from the C-termini of both LmHslU1 and LmHslU2. Truncations, Ala- and D-scans of the C-terminal dodecapeptide of LmHslU2 (LmC12-U2) showed that five out of the six C-terminal residues of LmHslU2 are essential for binding to and activating HslV. Peptide cyclisation with a lactam bridge allowed shortening of the peptide without loss of potency. Finally, we found that dodecapeptides derived from HslU of other parasites and bacteria are able to activate LmHslV with similar or even higher efficiency. Importantly, using electron microscopy approaches, we observed that the activation of LmHslV was accompanied by a large conformational remodeling, which represents a yet unidentified layer of control of HslV activation.



