Laurent Gavara
associate professor, ENSM
Laurent Gavara completed his M. Sci. in 2005 in Montpellier and pursued his graduate studies under the supervision of Pr. Jean-Pierre Hénichart and Pr. Benoit Rigo at the University of Lille, where he received his Ph.D. in 2008. He conducted a first postdoctoral work for 2 years in Clermont-Ferrand in the Pascal Moreau group and then moved to a second postdoctoral position for 1 year in Fort Worth, US, with the Pr. Jean-Luc Montchamp. In 2012, he joined the group of Muriel Amblard at the faculty of pharmacy of Montpellier as research fellow. One year later, he reached an associate professor position in the same group. His research interests are focused on the design and the synthesis of small heterocyclic molecules. He is currently working to fight the bacterial resistance thank to the inhibition of key bacterial enzymes.
Contact:
laurent.gavara@umontpellier.fr
+33 (0)4 48 79 21 80
5 major publications :
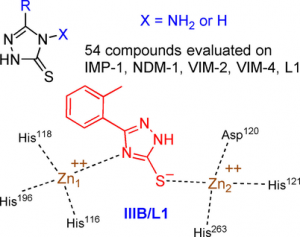
L. Gavara, F. Verdirosa, L. Sevaille, A. Legru, G. Corsica, L. Nauton, P. Sandra Mercuri, F. Sannio, F. De Luca, M. Hadjadj, G. Cerboni, Y. Vo Hoang, P. Licznar-Fajardo, M. Galleni, J.-D. Docquier, J.-F. Hernandez, 1,2,4-Triazole-3-thione analogues with an arylakyl group at position 4 as metallo-β-lactamase inhibitors. Bioorg. Med. Chem. 2022, 72, 116964.
F. Verdirosa, L. Gavara, L. Sevaille, G. Tassone, G. Corsica, A. Legru, G. Feller, G. Chelini, P. Sandra Mercuri, S. Tanfoni, F. Sannio, M. Benvenuti, G. Cerboni, F. De Luca, E. Bouajila, Y. Vo Hoang, P. Licznar-Fajardo, M. Galleni, C. Pozzi, S. Mangani, J.-D. Docquier, J.-F. Hernandez, 1,2,4-Triazole-3-Thione Analogues with a 2-Ethylbenzoic Acid at Position 4 as VIM-type Metallo-β-Lactamase Inhibitors. ChemMedChem 2022, e202100699.
L. Gavara, A. Legru, F. Verdirosa, L. Sevaille, L. Nauton, G. Corsica, P. Sandra Mercuri, F. Sannio, G. Feller, R. Coulon, F. De Luca, G. Cerboni, S. Tanfoni, G. Chelini, M. Galleni, J.-D. Docquier, J.-F. Hernandez, 4-Alkyl-1,2,4-triazole-3-thione analogues as metallo-β-lactamase inhibitors. Bioorg. Chem. 2021, 113, 105024.
A. Legru, F. Verdirosa, J.-F. Hernandez, G. Tassone, F. Sannio, M. Benvenuti, P.-A. Conde, G., C. A. Thomas, M. W. Crowder, M. Dillenberger, K. Becker, C. Pozzi, S. Mangani, J.-D. Docquier, L. Gavara, 1,2,4- riazole-3-thione compounds with a 4-ethyl alkyl/aryl sulfide substituent are broad-spectrum metallo-b-lactamase inhibitors with re-sensitization activity. Eur. J. Med. Chem. 2021, 226, 113873.
L. Gavara, L. Sevaille, F. De Luca, P. Mercuri, C. Bebrone, G. Feller, A. Legru, G. Cerboni, S. Tanfoni, D. Baud, G. Cutolo, B. Bestgen, G. Chelini, F. Verdirosa, F. Sannio, C. Pozzi, M. Benvenuti, K. Kwapien, M. Fischer, K. Becker, J.-M. Frère, S. Mangani, N. Gresh, D. Berthomieu, M. Galleni, J.-D. Docquier, J.-F. Hernandez, 4-Amino-1,2,4-triazole-3-thione-derived Schiff bases as metallo-β-lactamase inhibitors. Eur. J. Med. Chem. 2020, 208, 112720.