LUC BRUNEL
CNRS EnGIneer
Luc BRUNEL graduated from a DEA in heterocycle chemistry, polymers and catalysis obtained at the University of Montpellier in 1997. After several experiences in the private sector, since 2003 he has been working as a CNRS engineer in biomolecule development.
He works on both the SynBio3 platform providing biomolecules of biological interest and pharmaceuticals, as responsible for syntheses and purifications in large quantities, but also within the research team of the laboratory to carry out certain precursor projects.
.Luc has very solid skills in the fields of peptide synthesis in solution and on solid support, with a specialty in the field of sulfo and phosphopeptides.
.He also has 20 years of expertise in the implementation, development of new methods and scale-up in purification by preparative HPLC.
Luc obtained the CNRS Crystal medal in 2015.
Contact:
luc.brunel@umontpellier.fr
+33 (0)4 48 79 22 37
5 major publications :
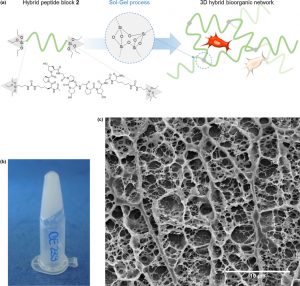
A Collagen-Mimetic Organic-Inorganic Hydrogel for Cartilage Engineering. Valot L, Maumus M, Brunel L, Martinez J, Amblard M, Noël D, Mehdi A, Subra G. Gels. 2021 Jun 15;7(2):73. doi: 10.3390/gels7020073
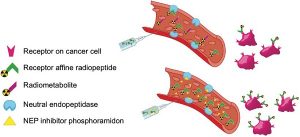
In Vivo Stabilization of a Gastrin-Releasing Peptide Receptor Antagonist Enhances PET Imaging and Radionuclide Therapy of Prostate Cancer in Preclinical Studies. Chatalic KL, Konijnenberg M, Nonnekens J, de Blois E, Hoeben S, de Ridder C, Brunel L, Fehrentz JA, Martinez J, van Gent DC, Nock BA, Maina T, van Weerden WM, de Jong M. Theranostics. 2016 Jan 1;6(1):104-17. doi: 10.7150/thno.13580. eCollection 2016.
Gastrin releasing peptide receptor-directed radioligands based on a bombesin antagonist: synthesis, (111)in-labeling, and preclinical profile. Marsouvanidis PJ, Nock BA, Hajjaj B, Fehrentz JA, Brunel L, M’Kadmi C, van der Graaf L, Krenning EP, Maina T, Martinez J, de Jong M. J Med Chem. 2013 Mar 28;56(6):2374-84. doi: 10.1021/jm301692p. Epub 2013 Mar 8.
An innovative strategy for sulfopeptides analysis using MALDI-TOF MS reflectron positive ion mode. Cantel S, Brunel L, Ohara K, Enjalbal C, Martinez J, Vasseur JJ, Smietana M. Proteomics. 2012 Aug;12(14):2247-57. doi: 10.1002/pmic.201100525.
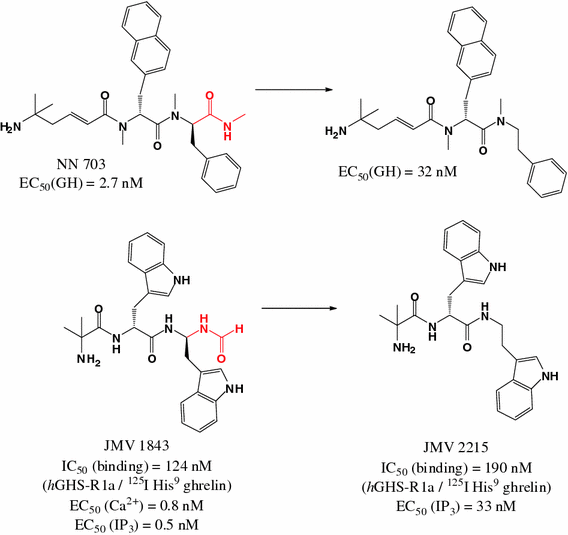
The 1,2,4-triazole as a scaffold for the design of ghrelin receptor ligands: development of JMV 2959, a potent antagonist. Moulin A, Brunel L, Boeglin D, Demange L, Ryan J, M’Kadmi C, Denoyelle S, Martinez J, Fehrentz JA. Amino Acids. 2013 Feb;44(2):301-14. doi: 10.1007/s00726-012-1355-2. Epub 2012 Jul 14. Review.