Abstract
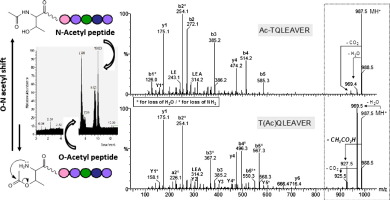
The detection of post-translational modifications (PTMs) of proteins is a matter of intensive research. Among all possible pitfalls that may lead to misidentifications, the chem. stability of modified peptides is scarcely questioned. Global proteomic studies devoted to protein acetylation are becoming popular. Thus, we were concerned about the intrinsic stability of O-acetylated peptides because of the O-N acyl transfer reactivity occurring when an amino moiety is present in the vicinity of the acylated hydroxyl group. Here, the behavior of isomeric O- and N-acetylated, N-terminal threonine-contg. peptides was explored in a std. proteomic workflow. We demonstrated a strong chem. instability of O-acetylation, which prevents its detection.