Bioorg. Chem., 2021, 115, 105218, https://doi.org/10.1016/j.bioorg.2021.105218
Drop, F. Jacquot, V. Canale, S. Chaumont-Dubel, M. Walczak, G. Satała, K. Nosalska, G.U. Mahoro, K. Słoczyńska, K. Piska, S. Lamoine, E. Pękala, N. Masurier, A.J. Bojarski, M. Pawłowski, J. Martinez, G. Subra, X. Bantreil, F. Lamaty, A. Eschalier, P. Marin, C. Courteix, P. Zajdel
Abstract
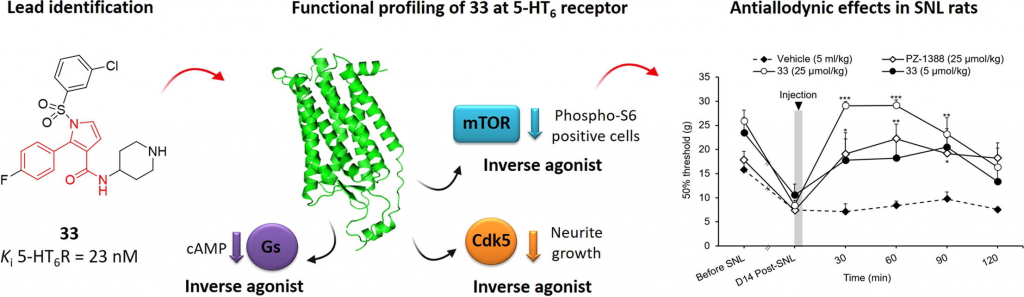
The diverse signaling pathways engaged by serotonin type 6 receptor (5-HT6R) together with its high constitutive activity suggests different types of pharmacological interventions for the treatment of CNS disorders. Non-physiological activation of mTOR kinase by constitutively active 5-HT6R under neuropathic pain conditions focused our attention on the possible repurposing of 5-HT6R inverse agonists as a strategy to treat painful symptoms associated with neuropathies of different etiologies. Herein, we report the identification of compound 33 derived from the library of 2-aryl-1H-pyrrole-3-carboxamides as a potential analgesic agent. Compound 33 behaves as a potent 5-HT6R inverse agonist at Gs, Cdk5, and mTOR signaling. Preliminary ADME/Tox studies revealed preferential distribution of 33 to the CNS and placed it in the low-risk safety space. Finally, compound 33 dose-dependently reduced tactile allodynia in spinal nerve ligation (SNL)-induced neuropathic rats.

