Eur. J. Med. Chem., 2023, 249, 115115, https://doi.org/10.1016/j.ejmech.2023.115115
P. Lagardère, R. Mustière, N. Amanzougaghene, S. Hutter, M. Casanova, J.-F. Franetich, S. Tajeri, A. Malzert-Fréon, S. Corvaisier, N. Azas, P. Vanelle, P. Verhaeghe, N. Primas, D. Mazier, N. Masurier, V. Lisowski
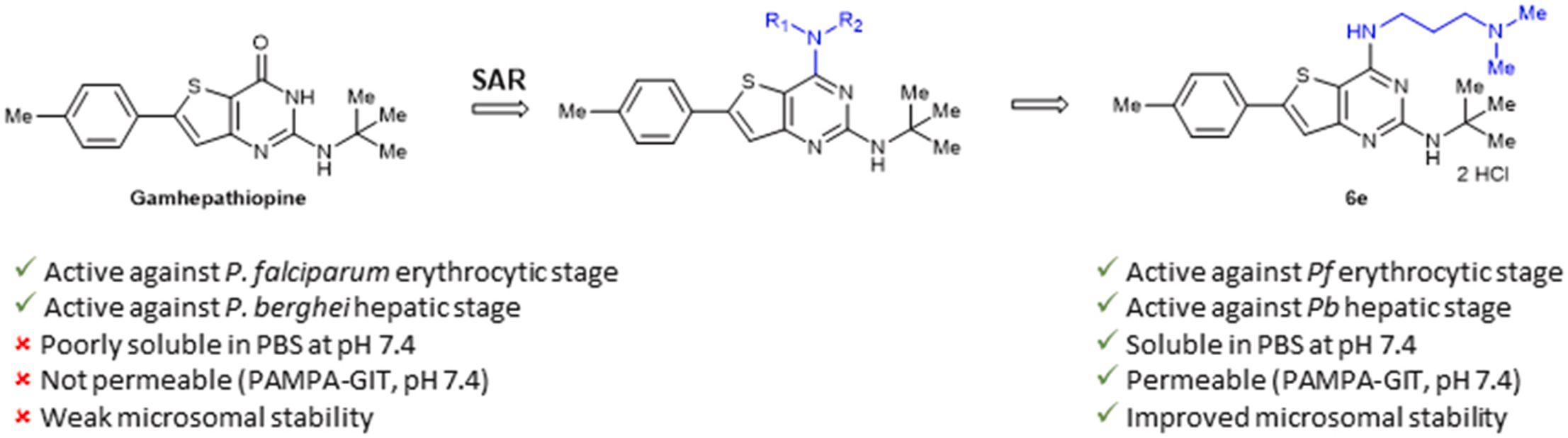
Abstract
The increasing number of Plasmodium falciparum strains resistant to current treatments justifies the urgent need to discover new compounds active on several stages of the parasite development. Based on the structure of Gamhepathiopine, a 2-tert-butylaminothieno[3,2-d]pyrimidin-4(3H)-one previously identified for its dual activity against the sexual and asexual stages of P. falciparum, 25 new 4-amino-substituted analogues were synthesized and evaluated on the erythrocytic and hepatic stages of Plasmodium. A promising compound, N2-(tert-butyl)-N [4]-(3-(dimethylamino)propyl)-6-(p-tolyl)thieno[3,2-d]pyrimidine-2,4-diamine, showed improved physicochemical properties, intestinal permeability (PAMPA model) and microsomal stability compared to Gamhepathiopine, while maintaining a good antiplasmodial activity on the erythrocytic stage of P. falciparum and on the hepatic stage of P. berghei.

