Eur. J. Med. Chem., 2023, 261, 115873, https://doi.org/10.1016/j.ejmech.2023.115873
P. Lagardère, R. Mustière, N. Amanzougaghene, S. Hutter, M. Casanova, J.-F. Franetich, S. Tajeri, A. Malzert-Fréon, S. Corvaisier, M. Since, N. Azas, P. Vanelle, P. Verhaeghe, N. Primas, D. Mazier, N. Masurier, V. Lisowski
Abstract
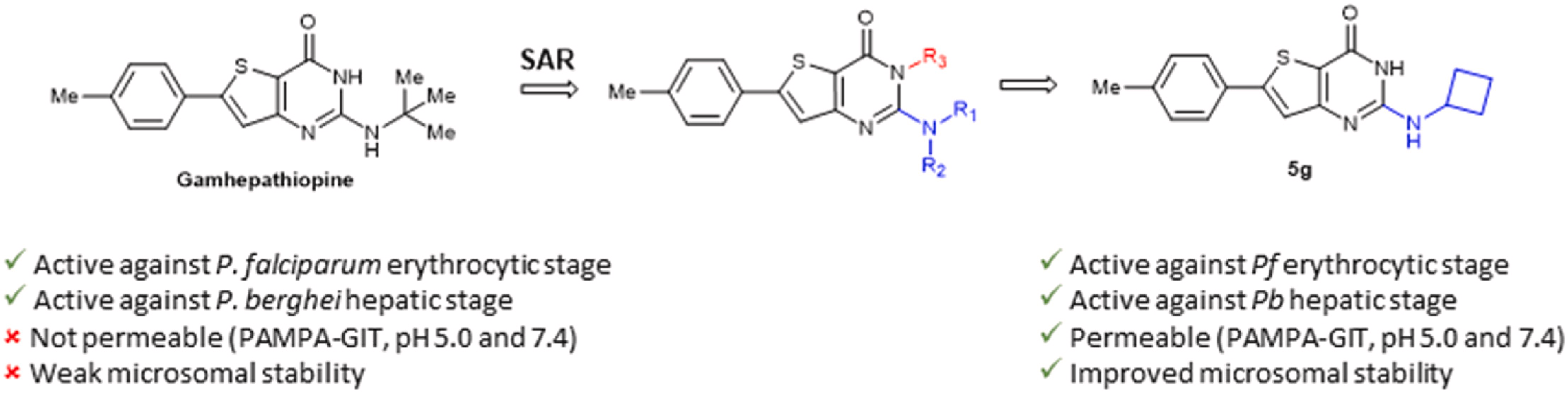
Based on the structure of a previously identified hit, Gamhepathiopine 1, which showed promising antiplasmodial activity, but poor microsomal stability, several strategies were investigated to improve the metabolic stability of the compounds. This included the introduction of fluorine or deuterium atoms, as well as carbocyclic groups. Among the new compounds, the 2-aminocyclobutyl derivative 5g demonstrated enhanced microsomal stability compared to compound 1, while retaining antiplasmodial activity against erythrocytic and hepatic stages of Plasmodium, without significant cytotoxicity against primary hepatocytes.

