Abstract
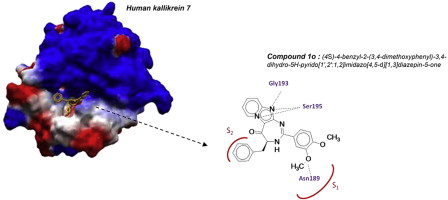
The human tissue kallikrein-7 (KLK7) is a chymotryptic serine protease member of tissue kallikrein family. KLK7 is involved in skin homeostasis and inflammation. Excess of KLK7 activity is also assocd. with tumor metastasis processes, esp. in ovarian carcinomas, prostatic and pancreatic cancers. Development of Kallikrein 7 inhibitors is thus of great interest in oncol. but also for treating skin diseases. Most of the developed synthetic inhibitors present several drawbacks such as poor selectivity and unsuitable physico-chem. properties for in vivo use. Recently, the authors described a practical sequence for the synthesis of imidazopyridine-fused [1,3]-diazepines. Here, the authors report the identification of pyrido-imidazodiazepinone core as a new potential scaffold to develop selective and competitive inhibitors of kallikrein-related peptidase 7. Structure-activity relationships (SAR), inhibition mechanisms and selectivity as well as cytotoxicity against selected cancer cell lines were investigated.