Abstract
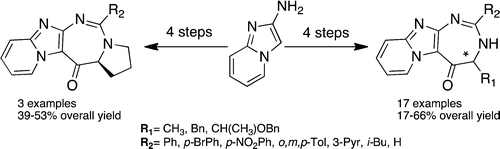
A series of 20 optically pure 3,4-dihydro-5H-pyrido[1′,2′:1,2]imidazo[4,5-d][1,3]diazepin-5-ones which form a new family of azaheterocycle-fused [1,3]diazepines were synthesized in four steps with 17–66% overall yields. The key step consists of a selective C-acylation reaction of easily accessible 2-aminoimidazo[1,2-a]pyridine at C-3.