Abstract
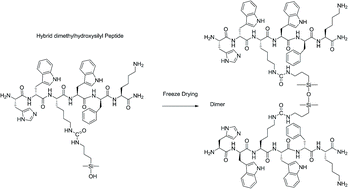
We developed a simple and straightforward way to dimerize unprotected peptide sequences that relies on a chemoselective condensation of hybrid peptides bearing a hydroxydimethylsilyl group at a chosen position (either C-ter, N-ter or side-chain linked) to generate siloxane bonds upon freeze-drying. Interestingly, the siloxane bond sensitivity to hydrolysis is strongly pH-dependent. Thus, we investigated the stability of siloxane dimers in different exptl. conditions. For that purpose, 29Si, 13C and 1H NMR spectra were recorded to accurately quantify the ratio of dimer/monomer. More interestingly, we showed that 1H resonances of the methylene and Me groups connected to the Si can be used as sensitive probes to monitor siloxane hydrolysis and to det. the half-lives of the dimers. Importantly, we showed that the dimers were rather stable at pH 7.4 (t1/2 ≈ 400 h) and we applied the dimerization strategy to bioactive sequences. Once optimized, three dimers of the growth hormone releasing hexapeptide (GHRP-6) were prepd. Interestingly, their pharmacol. evaluation revealed that the activity of the dimeric ligands could be switched from agonist to inverse agonist depending on the position of dimerization.