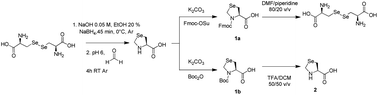
Abstract
In the search for new peptide ligands contg. selenium in their sequences, we investigated L-4-selenazolidine-carboxylic acid (selenazolidine, Sez) as a proline analog with the chalcogen atom in the γ-position of the ring. In contrast to proteinogenic selenocysteine (Sec) and selenomethionine (SeMet), the incorporation within a peptide sequence of such a non-natural amino acid has never been studied. There is thus a great interest in increasing the possibility of selenium insertion within peptides, esp. for sequences that do not possess a sulfur contg. amino acid (Cys or Met), by offering other selenated residues suitable for peptide synthesis protocols. Herein, we have evaluated selenazolidine in Boc/Bzl (Boc = tert-butoxycarbonyl) and Fmoc/tBu (Fmoc = 9-fluorenylmethoxycarbonyl) strategies through the synthesis of a model tripeptide, both in soln. and on a solid support. Special attention was paid to the stability of the Sez residue in basic conditions. Thus, generic protocols have been optimized to synthesize Sez-contg. peptides, through the use of an Fmoc-Xxx-Sez-OH dipeptide unit. As an example, a new analog of the vasopressin receptor-1A antagonist was prepd., in which Pro was replaced with Sez [3-(4-hydroxyphenyl)-propionyl-D-Tyr(Me)-Phe-Gln-Asn-Arg-Sez-Arg-NH2]. Both proline and such pseudo-proline contg. peptides exhibited similar pharmacol. properties and endopeptidase stabilities indicating that the presence of the selenium atom has minimal functional effects. Taking into account the straightforward handling of Sez as a dipeptide building block in a conventional Fmoc/tBu SPPS strategy, this result suggested a wide range of potential uses of the Sez amino acid in peptide chem., for instance as a viable proline surrogate as well as a selenium probe, complementary to Sec and SeMet, for NMR and mass spectrometry anal. purposes.