Abstract
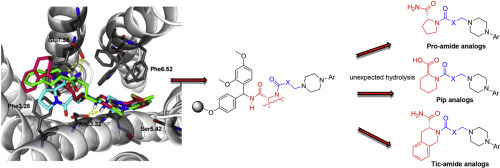
TA 47-membered library of novel long-chain arylpiperazines, which contained cyclic amino acid amides in the terminal fragment (pyrrolidine-2-carboxamide and 1,2,3,4-tetrahydroisoquinoline-3-carboxamide), was synthesized on Rink-amide resin and biol. evaluated for binding affinity for 5-HT7 and 5-HT1A receptors. Surprisingly, members of the designed series contg. piperidine-2-carboxamide fragments underwent hydrolysis, which occurred during the acidic treatment for release from the solid-support, to their resp. pipecolic acid analogs. Representative compds. from the library displayed high-to-low affinity for 5-HT7 (Ki = 18-3134 nM) and 5-HT1A (Ki = 0.5-6307 nM) sites. The possible interactions implicated in binding of the studied compds. to the 5-HT7 receptor were supported by mol. modeling. Research was also applied to support the exploration of the influence of the amide fragment, the length of alkylene spacer, and arylpiperazine substituents on the receptor’s affinity and selectivity.