Abstract
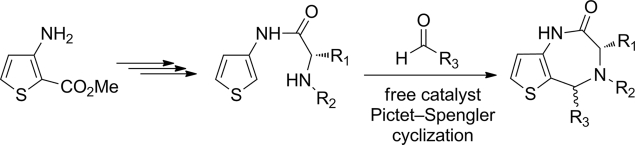
A series of 5-substituted thieno[3,2-e][1,4]diazepin-2-ones was synthesized in four steps from Me 3-aminothiophene-2-carboxylate. After the coupling of 3-aminothiophene with α-amino acids, the key final step that involves an uncatalyzed Pictet-Spengler reaction allowed the cyclization of the seven-membered diazepinone ring. The reaction was first optimized and then exemplified in three different series (phenylalanine, alanine and proline) that led to 24 target diazepinones, which includes 19 optically pure diastereomers.