Photochem. Photobiol. A, 2023, 435, 114287, https://doi.org/10.1016/j.jphotochem.2022.114287
M. Safir Filho, E. Santos Moraes, L. Camargo da Luz, F. da Silveira Santos, A. R. Martin, R. Benhida. L. G. Teixeira Alves Duarte, F. S. Rodembusch
Abstract
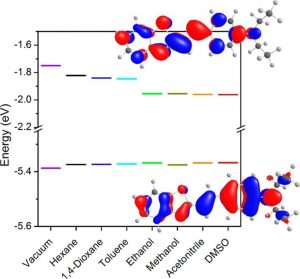
This study describes the synthesis, photophysical characterization, and TD-DFT calculation of a series of benzothiazole-based styryl fluorophores (F1–F5) harboring a D-π-A structure. The compounds were obtained through a simple and efficient synthetic route using low-cost starting materials. The investigation and analysis of their photophysical properties enable the assessment of the effect of four substituted benzothiazole moieties (A) and two types of π-conjugated frameworks (phenyl vs thienyl) in solution and in solid state. The fluorophores disclose absorption in the ultraviolet-blue region with a moderate solvatochromic effect and a single emission band located at the blue-green spectral range. In the solid state, the fluorophores showed emission in the green-orange regions with a relatively large Stokes shift. The systematic UV–vis solvatochromism study and theoretical predictions using the CPCM model reveals the increment in the dipole moment of the systems in an excited state with the solvent dielectric constant. These compounds showed high fluorescence quantum yield in solution and in solid-state and could be further exploited for solid-state lighting devices, or embedded in the design of biological dyes.