Ludovic Maillard
Assistant Professor, Faculty of Pharmacy, Montpellier University
Ludovic Maillard is Pharmacyst (Paris XI, 2002) and Chemical Engineer (ENSCP, Paris VI, 2002). After a Master Degree in organic chemistry (Paris VI, 2002), he did a PhD in bioorganic chemistry at the ICSN, in the group of Dr. B. Badet. Thereafter he joined the group of Prof. J. Robinson (OCI, Zurich, Switzerland) for a one-year post-doctoral fellow. He is actually Assistant Professor in medicinal chemistry.
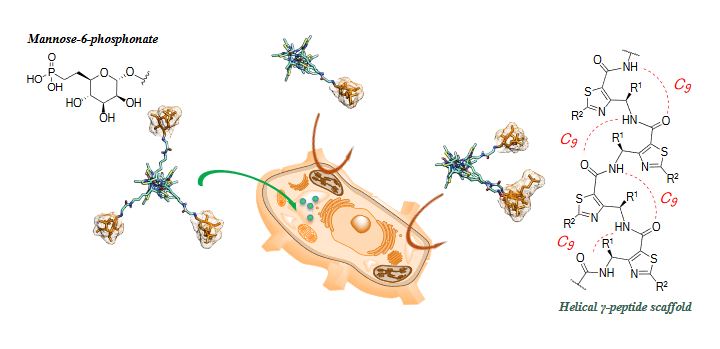
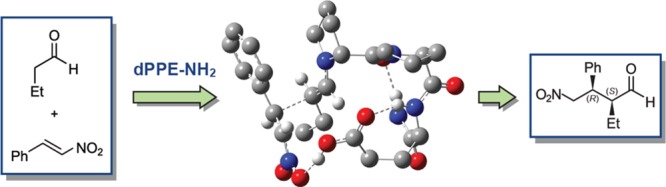
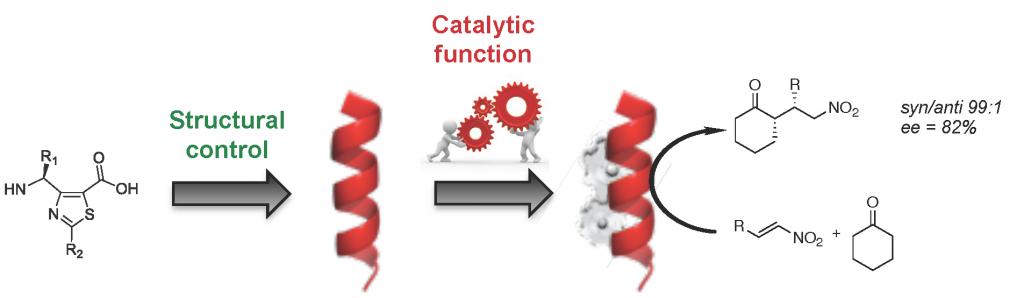
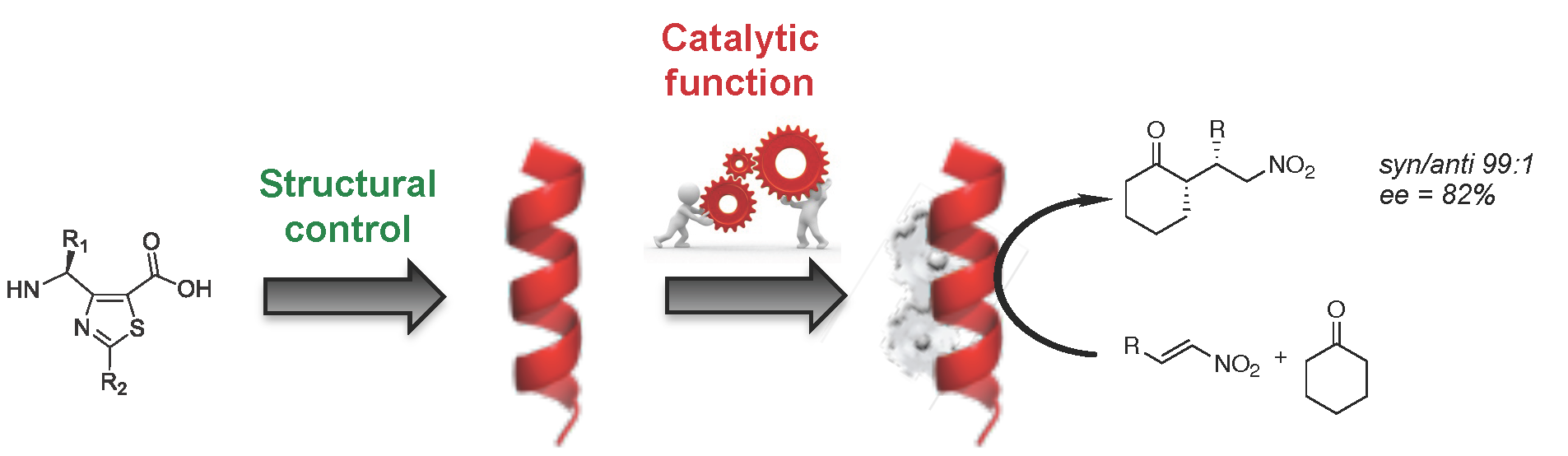
One of his major research interests is to develop and characterized conformationaly predictable molecular architectures named foldamers, which are constructed from heterocyclic γ-amino acids. In such a context, he has reported a short chemical route to access orthogonally protected thiazole-based γ-amino acids, which were used as building blocks for designing helical γ-peptide foldmers and antimicrobial peptides. These platforms are highly versatile and efforts are made to expand their applications toward cellular targeting and organo-catalysis.
Contact:
ludovic.maillard@umontpellier.Fr
+33 (0)4 48 79 21 84
5 major publications :
C. Bonnel, B. Legrand, M. Simon, J. Martinez, J.L. Bantignies, Y.K. Kang, E. Wenger, Francois Hoh, N. Masurier, L. T. Maillard* (2017)C9/12 Ribbon-Like Structures in Hybrid Peptides Alternating α- and Thiazole-Based γ-Amino Acids, Chem. Eur. J., 17584-17591
Bonnel C., Legrand B., Bantignies J.-L., Petitjean H., Martinez J., Masurier N. and Maillard L. T.* (2016) FT-IR and NMR structural markers for thiazole-based γ-peptide foldamers Org. Biomol. Chem. 8664-8669.
Mathieu L., Bonnel C., Masurier N., Maillard L. T.*, Martinez J. and Lisowski V. (2015) Cross-Claisen Condensation of N-Fmoc-Amino Acids – A Short Route to Heterocyclic γ-Amino Acids Eur. J. Org. Chem. 2262–2270.
Legrand B., Mathieu L., Lebrun A., Andriamanarivo S., Lisowski V., Masurier N., Zirah S., Kang Y. K., Martinez J., Maillard L.T.*, (2014) Thiazole-Based γ-Building Blocks as Reverse-Turn Mimetic to Design a Gramicidin S Analogue: Conformational and Biological Evaluation Chem. Eur. J. 6713-6720.
Mathieu L., Legrand B., Deng C., Vezenkov L., Wenger E., Didierjean C., Amblard M., Averlant-Petit MC., Masurier N., Lisowski V., Martinez J. and Maillard L.T.* (2013) Helical Oligomers of Thiazole-Based γ-Amino Acids: Synthesis and Structural Studies. Angew. Chem. Int. Ed. 52, 6006-6010.