CECILE ECHALIER
Assistant professor, Faculty of Pharmacy, University of Montpellier
Cécile Echalier is a researcher at the Institute of Biomolecules Max Mousseron and temporary lecturer at the University of Montpellier with expertise in biomaterials and chemical biology.
She studied organic and biomolecular chemistry at the University of Montpellier. There, she dedicated her PhD to the synthesis and evaluation of biomimetic materials for tissue engineering applications. Her PhD work was recognized with a L’Oréal-UNESCO For Women in Science fellowship. After her PhD, she joined the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany. In collaboration with GlaxoSmithKline, she developed chemical tools to elucidate the mode of action of drug candidates. In 2019, she joined the Stevens group at Imperial College London, UK. She was awarded a Scientia fellowship through the Marie Sklodowska-Curie Actions to develop an islets-on-chip platform with integrated biosensors in collaboration with the University of Oslo, Norway. She recently returned to Montpellier to resume her research on biomimetic hybrid materials for regenerative applications.
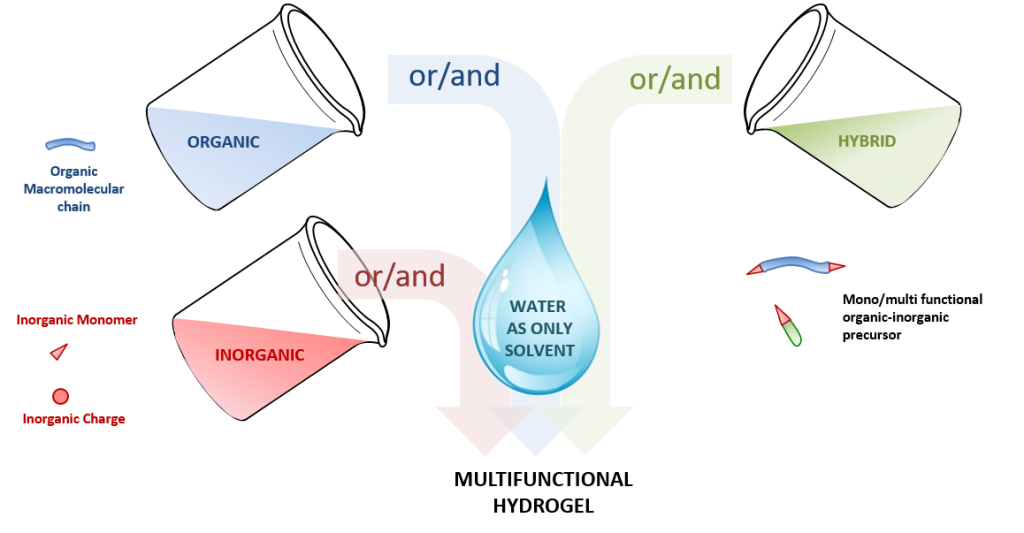
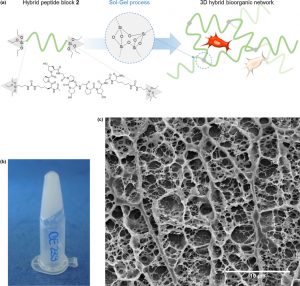
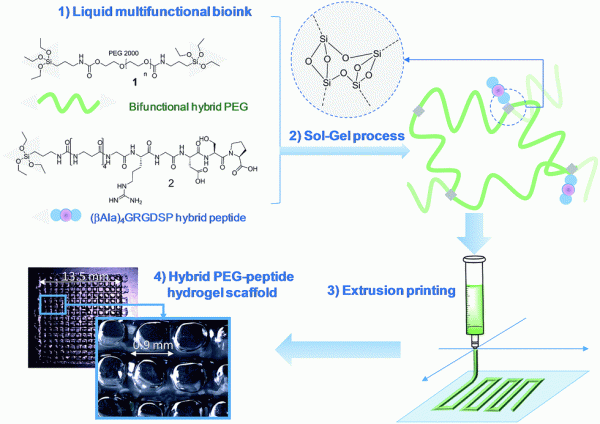
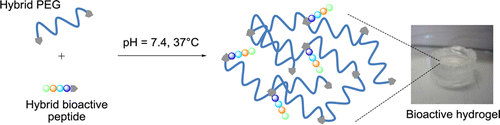
Her accomplishments include the development of a novel and modular method to prepare hydrogels by the sol-gel process. In particular, she demonstrated that the sol-gel process can be used both for crosslinking and covalent functionalization of hydrogels. She rendered the process fully biocompatible, enabling living cells encapsulation, and reported the very first example of sol-gel ink for extrusion-based 3D printing.
Her current research interests focus on the design of biomaterials that can recapitulate the complexity of native tissues for applications in tissue engineering. To achieve this, she develops new crosslinking chemistries and uses different 3D printing techniques.
5 major publications
(1) Montheil, T.; Simon, M.; Noël, D.; Mehdi, A.; Subra, G.*; Echalier, C*. Silylated Biomolecules: Versatile Components for Bioinks. Frontiers in Bioengineering and Biotechnology 2022, 10:888437. https://doi.org/10.3389/fbioe.2022.888437
(2) Echalier, C.; Rutkowska, A.; Kojic, A.; Thomson, D. W.; Edwards, L. J.; McKay, B. S. J.; Mülbaier, M.; Schultz, C.; Bergamini, G. AmTCO, a New Trans-Cyclooctene Derivative to Study Drug-Target Interactions in Cells. Chem. Commun. 2021, 57 (14), 1814–1817. https://doi.org/10.1039/D0CC06709A.
(3) Echalier, C.; Levato, R.; Mateos-Timoneda, M. A.; Castaño, O.; Déjean, S.; Garric, X.; Pinese, C.; Noël, D.; Engel, E.; Martinez, J.; Mehdi, A.; Subra, G. Modular Bioink for 3D Printing of Biocompatible Hydrogels: Sol–Gel Polymerization of Hybrid Peptides and Polymers. RSC Adv. 2017, 7 (20), 12231–12235. https://doi.org/10.1039/C6RA28540F.
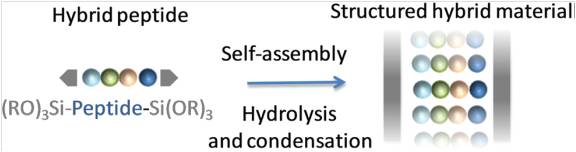
(4) Echalier, C.; Jebors, S.; Laconde, G.; Brunel, L.; Verdié, P.; Causse, L.; Bethry, A.; Legrand, B.; Van Den Berghe, H.; Garric, X.; Noël, D.; Martinez, J.; Mehdi, A.; Subra, G. Sol–Gel Synthesis of Collagen-Inspired Peptide Hydrogel. Materials Today 2017, 20 (2), 59–66. https://doi.org/10.1016/j.mattod.2017.02.001.
(5) Echalier, C.; Pinese, C.; Garric, X.; Van Den Berghe, H.; Jumas Bilak, E.; Martinez, J.; Mehdi, A.; Subra, G. Easy Synthesis of Tunable Hybrid Bioactive Hydrogels. Chem. Mater. 2016, 28 (5), 1261–1265. https://doi.org/10.1021/acs.chemmater.5b04881.