
Gilles Subra
Professor, Faculty of Pharmacy of Montpellier
Gilles leads his researches in the Institute of Biomolécules Max Mousseron (IBMM) in the field of peptide science. His main research topics are at the interface of chemistry and biology and notably concern methodologies for solid phase and combinatorial chemistry, design of chemical tools to enhance detection and quantification by mass spectrometry in biological media. More recently, he is interested in the design of peptide-based materials and polymers. The main applications concern the conception of multi-ligand nanoparticles for cancer targeting, the functionalisation of silicone medical devices and dressings. His most recent research is related to the design of biomimetic peptide based hydrogel used for 3D biofabrication and cell-based therapies.
Gilles Subra founded the solid phase and automated synthesis platform (SynBio3) of the IBMM whose mission is to develop solid supported reagents, linkers and methodologies for the synthesis of biomolecules.
Contact:
gilles.subra@umontpellier.fr
+33 411 75 96 06
5 major publications :
Ciccione J., Jia T., Coll J.-L., Parra K., Amblard M., Jebors S., Martinez J., Mehdi A., and Subra G. (2016). Unambiguous and Controlled One-Pot Synthesis of Multifunctional Silica Nanoparticles. Chem. Mater. 28, 885–889.
Echalier C., Pinese C., Garric X., Van Den Berghe H., Jumas Bilak E., Martinez J., Mehdi A., and Subra G. (2016). Easy Synthesis of Tunable Hybrid Bioactive Hydrogels. Chem. Mater. 28, 1261–1265.
Jebors S., Ciccione J., Al-Halifa S., Nottelet B., Enjalbal C., M’Kadmi C., Amblard M., Mehdi A., Martinez J., and Subra G. (2015). A New Way to Silicone-Based Peptide Polymers. Angew. Chem. Int. Ed. 54, 3778–3782.
Paramelle D., Subra G., Vezenkov L., Maynadier M., André C., Enjalbal C., Calmès M., Garcia M., Martinez J., and Amblard M. (2010). A Straightforward Approach for Cellular-Uptake Quantification. Angewandte Chemie International Edition 49, 8240–8243.
Paramelle D., Enjalbal C., Amblard M., Forest E., Heymann M., Cantel S., Geourjon C., Martinez J., and Subra G. (2011). Solid-Phase Cross-Linking (SPCL): A new tool for protein structure studies. PROTEOMICS 11, 1277–1286.
Neuropathic pain-alleviating activity of novel 5-HT6 receptor inverse agonists derived from 2-aryl-1H-pyrrole-3-carboxamide
Bioorg. Chem., 2021, 115, 105218, https://doi.org/10.1016/j.bioorg.2021.105218
Drop, F. Jacquot, V. Canale, S. Chaumont-Dubel, M. Walczak, G. Satała, K. Nosalska, G.U. Mahoro, K. Słoczyńska, K. Piska, S. Lamoine, E. Pękala, N. Masurier, A.J. Bojarski, M. Pawłowski, J. Martinez, G. Subra, X. Bantreil, F. Lamaty, A. Eschalier, P. Marin, C. Courteix, P. Zajdel
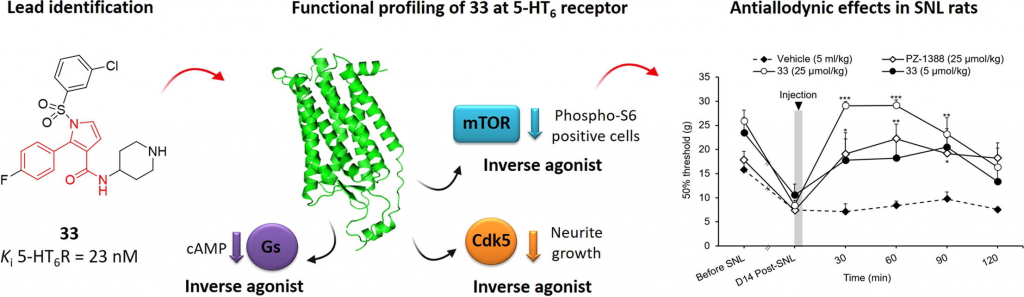
Abstract
The diverse signaling pathways engaged by serotonin type 6 receptor (5-HT6R) together with its high constitutive activity suggests different types of pharmacological interventions for the treatment of CNS disorders. Non-physiological activation of mTOR kinase by constitutively active 5-HT6R under neuropathic pain conditions focused our attention on the possible repurposing of 5-HT6R inverse agonists as a strategy to treat painful symptoms associated with neuropathies of different etiologies. Herein, we report the identification of compound 33 derived from the library of 2-aryl-1H-pyrrole-3-carboxamides as a potential analgesic agent. Compound 33 behaves as a potent 5-HT6R inverse agonist at Gs, Cdk5, and mTOR signaling. Preliminary ADME/Tox studies revealed preferential distribution of 33 to the CNS and placed it in the low-risk safety space. Finally, compound 33 dose-dependently reduced tactile allodynia in spinal nerve ligation (SNL)-induced neuropathic rats.
Simple and Specific Grafting of Antibacterial Peptides on Silicone Catheters
Advanced Healthcare Materials 5: 3067–3073, 2016. https://doi.org/10.1002/adhm.201600757
Abstract
To fight against nosocomial infection initiated by colonization of medical devices, a strategy enabling the direct and fast functionalization of silicone surfaces is proposed. This strategy proceeds in a site-specific way using original hybrid silylated antibacterial peptides. This safe and up-scalable method guarantees a covalent and robust immobilization with the correct orientation of the bioactive moiety. Importantly it also avoids multi-step chemical modifications of the surface or multi-layer polymer coatings. As proof of concept, antibacterial silicone catheter has been prepared whose immediate and long term efficiency is superior by comparison to similar silver-embedded materials.
Direct Synthesis of Peptide-Containing Silicones: A New Way to Bioactive Materials
Chemistry A European Journal, 26:12839-12845, 2020. https://doi.org/10.1002/chem.202001571
Abstract
A simple and efficient way to synthesize peptide-containing silicone materials is described. Silicone oils containing a chosen ratio of bioactive peptide sequences were prepared by acid-catalyzed copolymerization of dichlorodimethylsilane, hybrid dichloromethyl peptidosilane, and Si(vinyl)- or SiH-functionalized monomers. Functionalized silicone oils were first obtained and then, after hydrosilylation cross-linking, bioactive polydimethylsiloxane (PDMS)-based materials were straightforwardly obtained. The introduction of an antibacterial peptide yielded PDMS materials showing activity against Staphylococcus aureus. PDMS containing RGD ligands showed improved cell-adhesion properties. This generic method was fully compatible with the stability of peptides and thus opened the way to the synthesis of a wide range of biologically active silicones.
Silylated biomolecules: Versatile components for bioinks
Abstract
Physical hydrogels prepared from natural biopolymers are the most popular components for bioinks. However, to improve the mechanical properties of the network, in particular its durability for long-lasting tissue engineering applications or its stiffness for bone/cartilage applications, covalent chemical hydrogels have to be considered. For that purpose, biorthogonal reactions are required to allow the inclusion of living cells within the bioink reservoir before the 3D printing procedure. Interestingly, such reactions also unlock the possibility to further multifunctionalize the network, adding bioactive moieties to tune the biological properties of the resulting printed biomaterial. Surprisingly, compared to the huge number of studies disclosing novel bioink compositions, no extensive efforts have been made by the scientific community to develop new chemical reactions meeting the requirements of both cell encapsulation, chemical orthogonality and versatile enough to be applied to a wide range of molecular components, including fragile biomolecules. That could be explained by the domination of acrylate photocrosslinking in the bioprinting field. On the other hand, proceeding chemoselectively and allowing the polymerization of any type of silylated molecules, the sol-gel inorganic polymerization was used as a crosslinking reaction to prepare hydrogels. Recent development of this strategy includes the optimization of biocompatible catalytic conditions and the silylation of highly attractive biomolecules such as amino acids, bioactive peptides, proteins and oligosaccharides. When one combines the simplicity and the versatility of the process, with the ease of functionalization of any type of relevant silylated molecules that can be combined in an infinite manner, it was obvious that a family of bioinks could emerge quickly. This review presents the sol-gel process in biocompatible conditions and the various classes of relevant silylated molecules that can be used as bioink components. The preparation of hydrogels and the kinetic considerations of the sol-gel chemistry which at least allowed cell encapsulation and extrusion-based bioprinting are discussed.
3D high-precision melt electro written polycaprolactone modified with yeast derived peptides for wound healing
Abstract
In this study melt electro written (MEW) scaffolds of poly(ε-caprolactone) PCL are decorated with anti-inflammatory yeast-derived peptide for skin wound healing. Initially, 13 different yeast-derived peptides were screened and analyzed using both in vitro and in vivo assays.
The MEW scaffolds are functionalized with the selected peptide VLSTSFPPW (VW-9) with the highest activity in reducing pro-inflammatory cytokines and stimulating fibroblast proliferation, migration, and collagen production. The peptide was conjugated to the MEW scaffolds using carbodiimide (CDI) and thiol chemistry, with and without plasma treatment, as well as by directly mixing the peptide with the polymer before printing. The MEW scaffolds modified using CDI and thiol chemistry with plasma treatment showed improved fibroblast and macrophage penetration and adhesion, as well as increased cell proliferation and superior anti-inflammatory properties, compared to the other groups. When applied to full-thickness excisional wounds in rats, the peptide-modified MEW scaffold significantly enhanced the healing process compared to controls (p < 0.05). This study provides proof of concept for using yeast-derived peptides to functionalize biomaterials for skin wound healing.
One-Pot Synthesis of Bioinspired Peptide-Decorated Apatite Nanoparticles for Nanomedicine
Abstract
Hybrid organic-inorganic bio-inspired apatite nanoparticles (NPs) are attractive for biomedical applications and especially in nanomedicine. Unfortunately, functionalized apatite NPs were so far synthesized by multistep processes and often yielding broad particle size distributions. In addition, although the adsorption of drugs or antibodies on apatite NPs surfaces was reported, it was generally associated with uncontrolled drug loading. All these limitations have restricted their actual applications in nanomedicine. Besides, very few attempts at exposing bioactive peptides on apatite NPs have been made. In this work, we report an original one-pot synthesis of well-defined bioactive hybrid NPs composed of a mineral core of bioinspired apatite surrounded by an organic corona of bioactive peptides. Dual stabilizing-bioactive agents, phosphonated PEG-peptide conjugates, were prepared and directly used during apatite precipitation (i) to form the organic corona during apatite precipitation, driving the size and shape of resulting hybrid NPs with colloidal stabilization and (ii) to expose peptide moieties (RGD or YIGSR sequences) at the NPs periphery in view of conferring additional surface properties to enhance their interaction with cells. Here, we demonstrate the success of this approach, we fully characterized the functionalized NPs by FTIR, Raman, XRD, solid and liquid state NMR, TEM and DLS, and we followed their interaction with fibroblast cells, unveiling a synergistic proliferative effect.
Sol-gel polymerization of silylated amino acids around a protein template yields selective biomimetic imprints
Raquel Gutiérrez-Climente, Giang Ngo, Margaux Clavié, Jérémie Gouyon, Yoann Ladner, Pascal Etienne, Pascal Dumy, Catherine Perrin, Ahmad Mehdi, Pierre Martineau, Martine Pugnière and Gilles Subra
Abstract
The development of artificial receptors able to selectively recognize a target protein is of particular interest in separation, diagnostics, and therapeutics fields. Herein, we disclose a method to prepare biomimetic and functionalized protein imprints in biocompatible conditions avoiding any protein denaturation. For that purpose, a set of different hybrid silylated amino acid derivatives were synthesized and used without tetraethyl orthosilicate to prepare our molecularly imprinted polymers, allowing to reduce to a minimum of the silicon amount, in order to obtain imprints made almost entirely of amino acids to mimic paratope surfaces of antibodies. Such functional building blocks were polymerized on the surface of magnetic silica nanoparticles at pH 8.5 in ultrapure water in the presence of two globular proteins: cytochrome C or lysozyme. The resulting imprinted hybrid materials were evaluated for their adsorption capacity, specificity, and selectivity by quartz-crystal microbalance with dissipation and magnetic enzyme-linked immunosorbent assay (ELISA) assays. High imprinting factors of 8.7 were measured for these biomimetic hybrid materials (corresponding to approximately 4000 and 450 ng of protein per cm2 immobilized on molecularly imprinted polymers and non-imprinted polymer nanoparticles, respectively), representing a significant breakthrough in sol-gel-based molecular imprinting materials. Moreover, competition experiments performed by magnetic ELISA (mELISA) show very good specificity of our imprints at the usual concentrations of ELISA measurements.
Development of Amino Acids Functionalized SBA-15 for the Improvement of Protein Adsorption
Molecules 2021, 26(19), 6085; https://doi.org/10.3390/molecules26196085
Raquel Gutiérrez-Climente, Margaux Clavié, Jérémie Gouyon, Giang Ngo, Yoann Ladner, Pascal Etienne, Pascal Dumy, Pierre Martineau, Martine Pugnière, Catherine Perrin, Gilles Subra and Ahmad Mehdi
Abstract
Ordered mesoporous materials and their modification with multiple functional groups are of wide scientific interest for many applications involving interaction with biological systems and biomolecules (e.g., catalysis, separation, sensor design, nano-science or drug delivery). In particular, the immobilization of enzymes onto solid supports is highly attractive for industry and synthetic chemistry, as it allows the development of stable and cheap biocatalysts. In this context, we developed novel silylated amino acid derivatives (Si-AA-NH2) that have been immobilized onto SBA-15 materials in biocompatible conditions avoiding the use of toxic catalyst, solvents or reagents. The resulting amino acid-functionalized materials (SBA-15@AA) were characterized by XRD, TGA, EA, Zeta potential, nitrogen sorption and FT-IR. Differences of the physical properties (e.g., charges) were observed while the structural ones remained unchanged. The adsorption of the enzyme lysozyme (Lyz) onto the resulting functionalized SBA-15@AA materials was evaluated at different pHs. The presence of different functional groups compared with bare SBA-15 showed better adsorption results, for example, 79.6 nmol of Lyz adsorbed per m2 of SBA-15@Tyr compared with the 44.9 nmol/m2 of the bare SBA-15.
Hydrocarbon-Stapled Peptide Based-Nanoparticles for siRNA Delivery.
Nanomaterials (Basel). 2020 Nov 25;10(12):2334. doi: 10.3390/nano10122334
Simon M, Laroui N, Heyraud M, Laconde G, Ali LMA, Bourbiaux K, Subra G, Vezenkov LL, Legrand B, Amblard M, Bettache N.
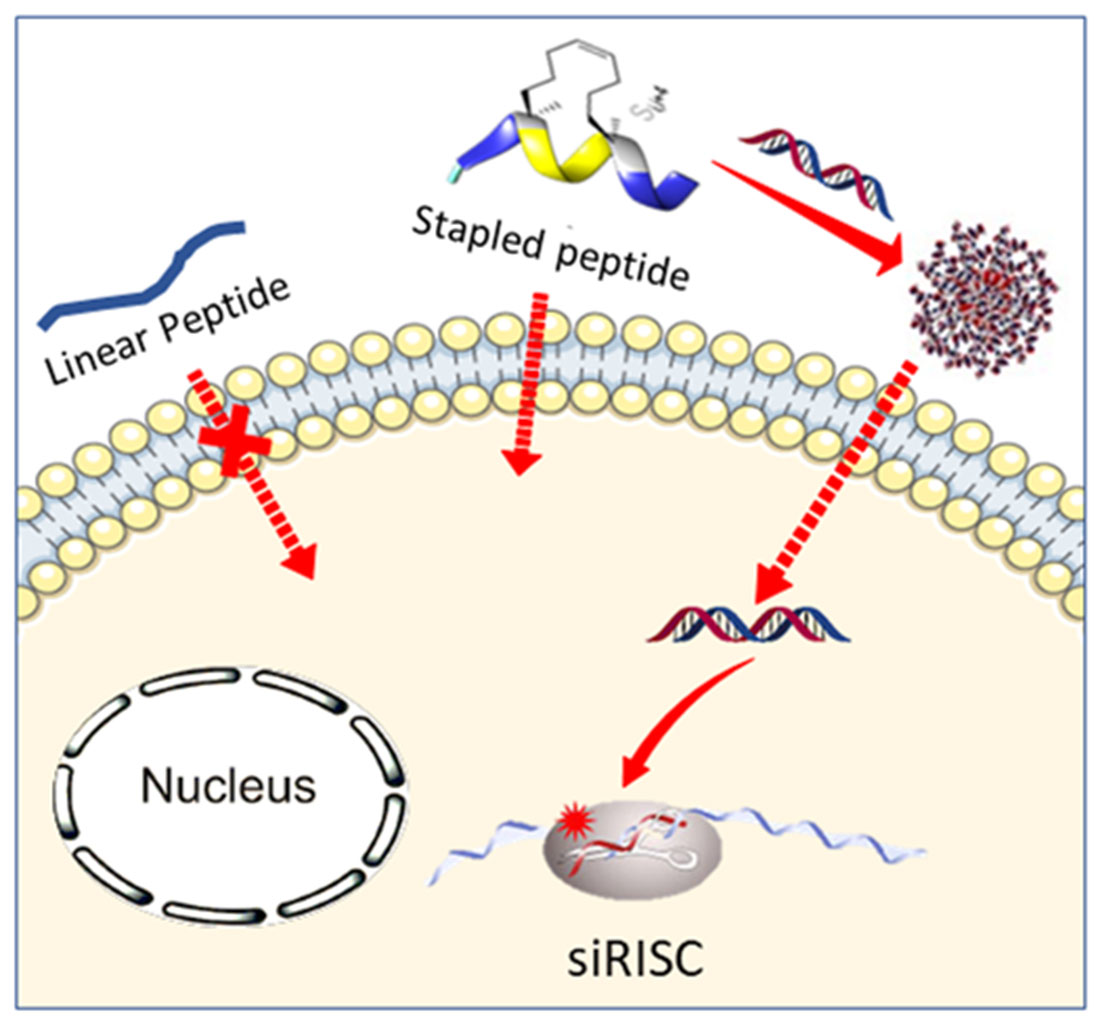
Abstract
Small interfering RNAs (siRNAs) are promising molecules for developing new therapies based on gene silencing; however, their delivery into cells remains an issue. In this study, we took advantage of stapled peptide technology that has emerged as a valuable strategy to render natural peptides more structured, resistant to protease degradation and more bioavailable, to develop short carriers for siRNA delivery. From the pool of stapled peptides that we have designed and synthesized, we identified non-toxic vectors that were able to efficiently encapsulate siRNA, transport them into the cell and induce gene silencing. Remarkably, the most efficient stapled peptide (JMV6582), is composed of only eight amino-acids and contains only two cationic charges.
Nano-assemblies with core-forming hydrophobic polypeptide via polymerization-induced self-assembly (PISA)
Polym. Chem., 2021,12, 113-121 DOI: 10.1002/macp.202000311
Dao T, Vezenkov L, Subra G, Ladmiral V, Semsarilar M.
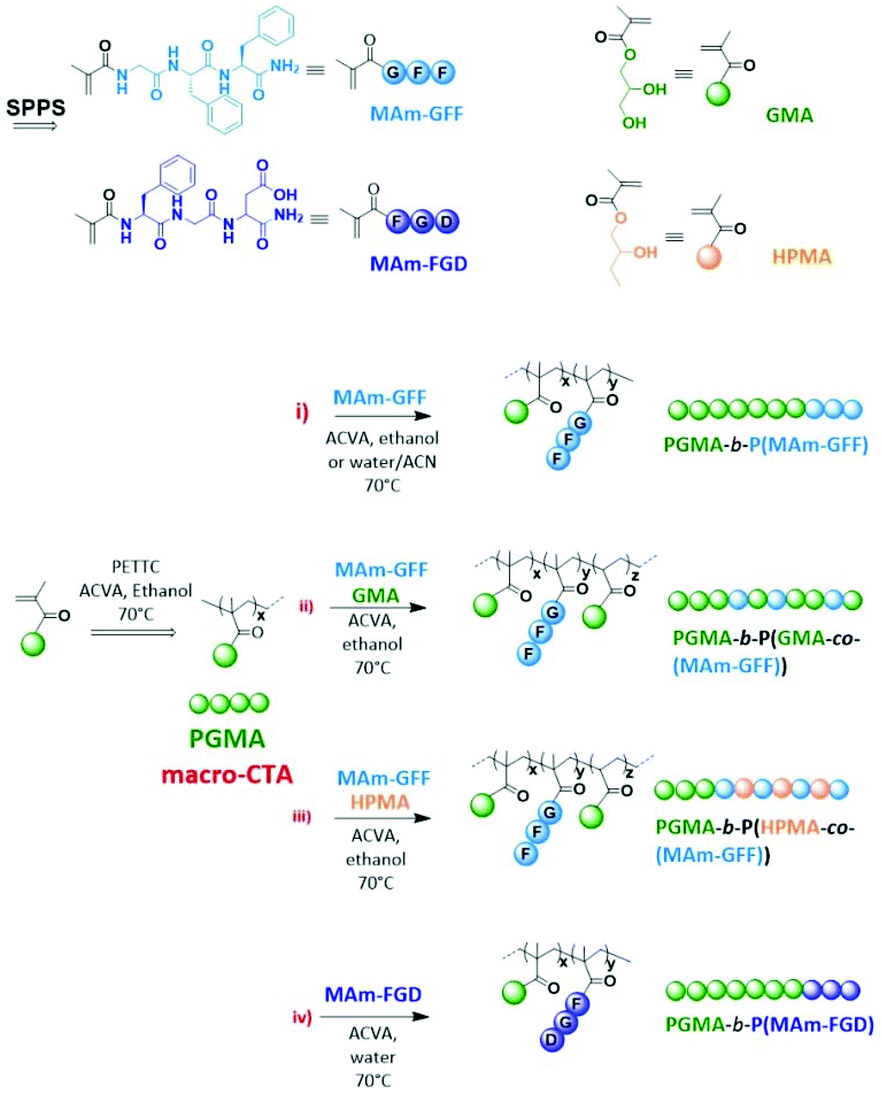
Abstract
The aim of this study is to produce self-assembled structures with hydrophobic polypeptide cores via Reversible Addition–Fragmentation chain Transfer (RAFT) – mediated Polymerisation-Induced Self-Assembly (PISA). Hydrophilic poly(glycerol monomethacrylate) macromolecular chain transfer agents (PGMA mCTAs) were used to polymerize the self-assembling peptide monomers, resulting in the formation of diblock copolymer nano objects. Methacrylamide derivatives containing self-assembling tripeptides MAm-GFF (MAm-Gly-Phe-Phe-NH2) and MAm-FGD (MAm-Phe-Gly-Asp-NH2) were used as hydrophobic monomers. The self-assembling behaviours of these monomers mainly derive from the interactions of the phenylalanine residues, however their difference in hydrophobicity required different polymerization conditions. MAm-GFF was polymerized in the presence of organic solvent (ethanol or acetonitrile), under either dispersion or emulsion polymerization, while MAm-FGD was polymerized under aqueous dispersion conditions. PGMA-b-P(MAm-FGD) obtained from aqueous PISA typically formed fibrous structures while a range of morphologies such as fibre-, flake-, and leaf-like or spherical vesicles were obtained for PGMA-b-P(MAm-GFF) depending on the copolymer composition and solvent used. In all cases the peptides self-assembling core had a crucial influence on the final morphologies.



