Funding: ANR-18-CE19-023-02
January 2019- June 2022
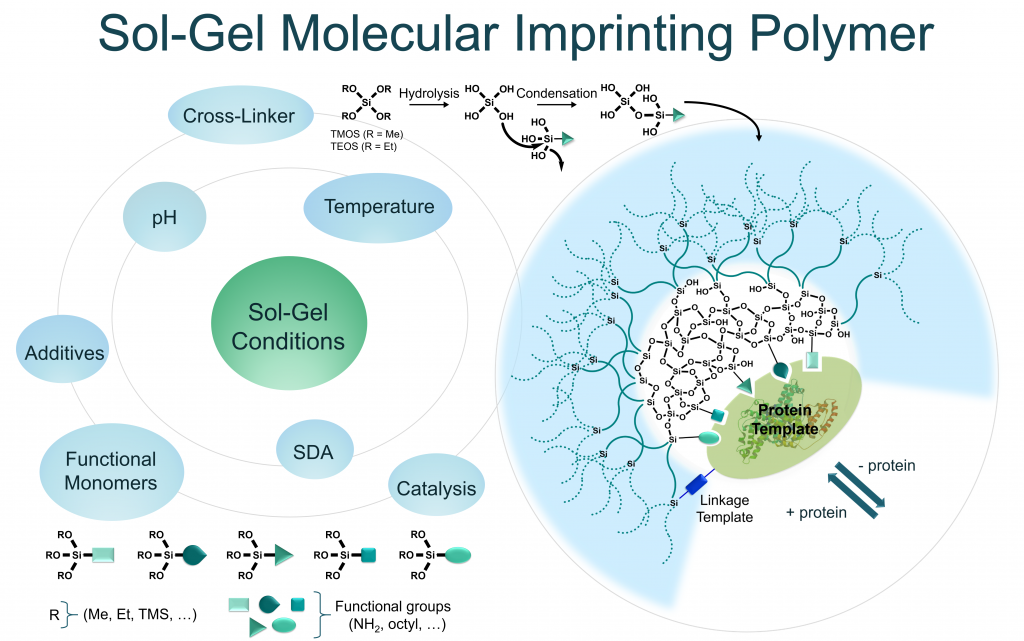
The concept of ‘molecular imprinting’ emerged in the 1930’s and thanks to its conceptual simplicity, it always attracted a tremendous interest of many scientific communities. The goal is to obtain the hollow shape of a molecule (i.e. ‘template ‘) in a three-dimensional matrix. The template is then removed from the material to give the empty cavities, which are now able to selectively capture the initial object (i.e. the template molecule). These molecular imprints were obtained either inside a material (e.g. hydrogel, macroporous matrix etc.) or on the surface of a substrate. Both organic or inorganic polymerization of monomeric precursors have been used to prepare imprinted materials. However, most of precursors (monomers) commonly used for molecular imprinting are organic ones that react between them by free radical polymerization.
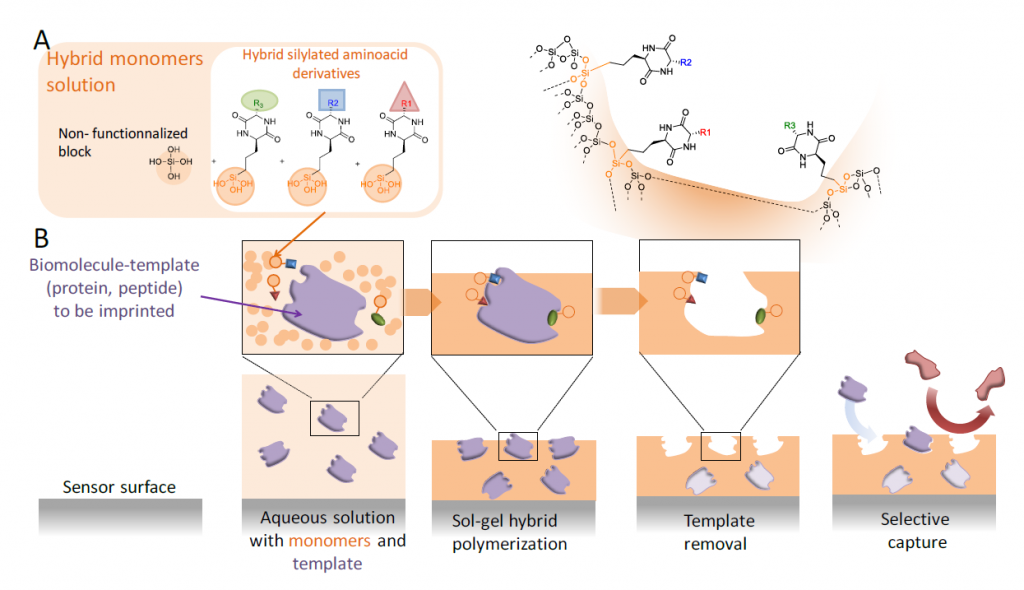
In this sense, the objective of Peptimprint is to set-up a ground-breaking technology to prepare highly specific tridimensional and functionalized imprints of biomolecules (peptides, proteins) by template-assembly, but in this case following the inorganic polymerization by sol-gel process. For that, a variety of original hybrid building blocks mimicking amino acids (diketopiperazine) will be synthetised and developed to be used as precursors (functional monomers) in the MIP synthesis. Unfunctionalized blocks (e.g. tetraethoxysilane (TEOS), methyltriethoxysilane, dimethyldiethoxysilane, bis(triethoxysilyl) ethylene, mono-silylated PEG or bis-silylated PEG) will be also used concomitantly to create the network. Once the template molecule removed, the hollow cavities will be able to capture the biomolecule which was previously imprinted with very high selectivity and affinity. Indeed, unlike classical molecular imprint polymers (MIPs), the resulting inorganic/bioorganic hybrid material will recapitulate not only the shape but also the complementary functions of the biomolecular template.
This project is developed in collaboration with team “Analytic Sciences of Biomolecules & Molecular Modelling” of the Institute of Biomolecules Max Mousseron (IBMM), the team “Molecular Chemistry and Solid-state Organization (CMOS)” of the Institute Charles Gerhardt (ICG), the team “Optomicrofluidique (POMM)” from the Charles Coulomb laboratory of the University of Montpellier, and the team interactions of the “Institut de Recherche en Cancérologie de Montpellier” (IRCM).
THE TEAM

PASCAL ETIENNE

PIERRE MARTINEAU

MARTINE PUGNIÈRE

RAQUEL GUTIÉRREZ-CLIMENTE

JÉRÉMIE GOUYON

GIANG NGO

MARGAUX CLAVIÉ
| Date | Congress | Type presentation | Title presentation |
| 15-18 June 2021 | Balard Chemistry Conferences 2021 | Oral Communication | Biomimetic protein imprinting on magnetic particles using amino acid-based hybrid blocks |
| 18 June 2021 | aesap (Association des Enseignants de Sciences Analytiques de Pharmacie | Flash Communication | Silylated amino acids as hybrid precursors for protein-biomimetic surface coating: application to electrophoresis separation |
| 22-24 June 2021 | Affinity 2021 | Flash Communication | Amino acid-based hybrid blocks for biomimetic protein imprinting |
| 5-7 July 2021 | ISOS-2021 The 19th International Symposium on Silicon Chemistry | Flash Communication | |
| 14 July 2021 | e-MSB 2021 Boston | Oral Communication | Silylated amino acids as hybrid precursors for protein-biomimetic surface coating: application to electrophoresis separation |
| 5-7 October 2021 | SEP21- 14ème Congrès Francophone sur les Sciences Séparatives et les Couplages de l’AFSEP | Oral communication | Silylated amino acids as hybrid precursors for protein-biomimetic surface coating: application to electrophoresis separation
|
| 28-29 October 2021 | JMJC2021 9èmes Journées Méditerranéennes des Jeunes Chercheur(e)s | Poster Communication | Applications of silylated amino acid in the development of hybrid materials |
| Oral Communication | Silylated amino acids as hybrid precursors for molecularly imprinted polymers |
||
| 2-3 November 2021 | QSense User Days – Biolin Scientific | Oral Communication | Using QCM-D to study the recognition of molecularly imprinted polymer particles toward their target proteins |