Nicolas masurier
Professor, Faculty of Pharmacy, Montpellier University
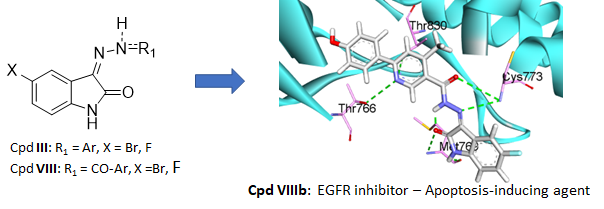
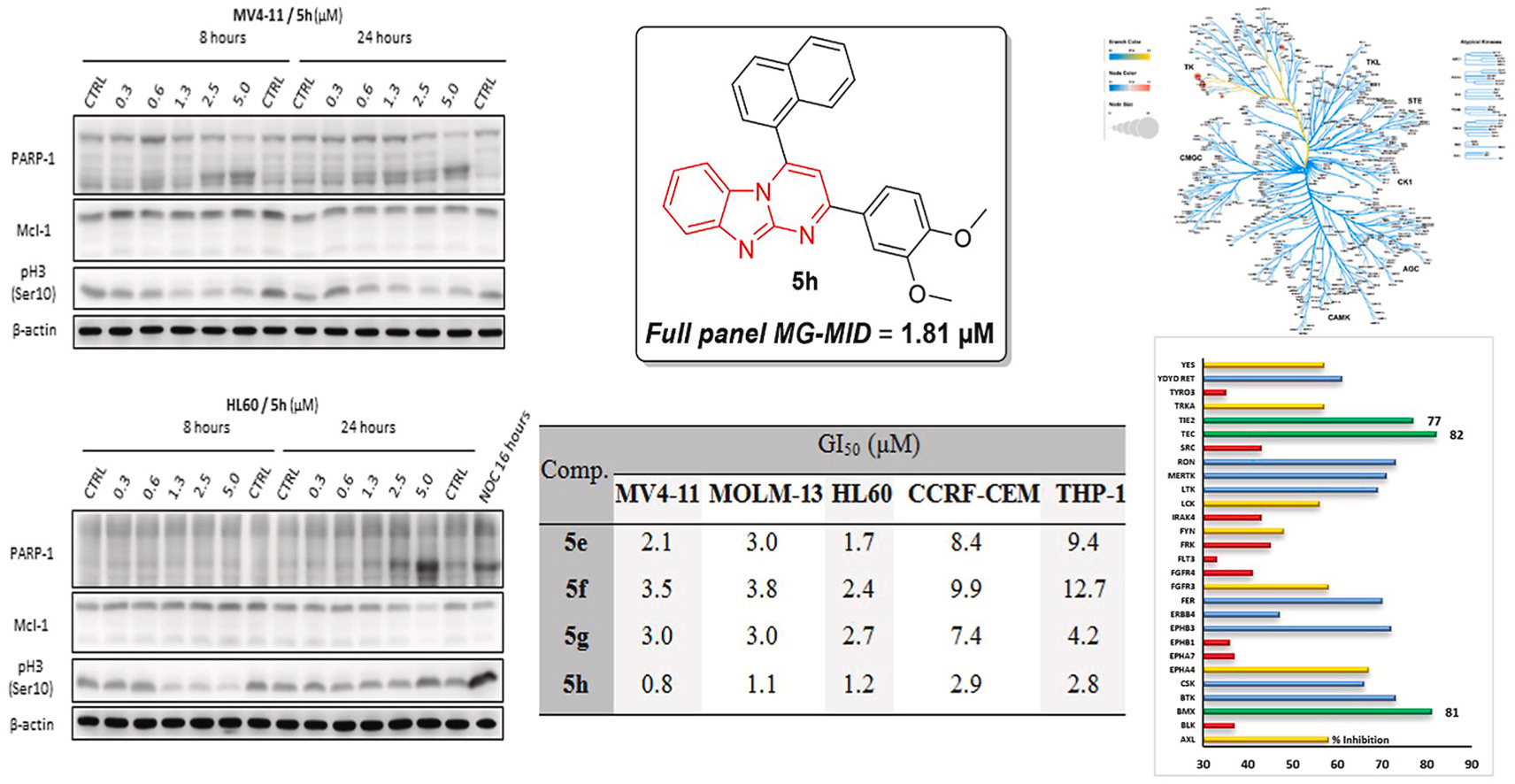
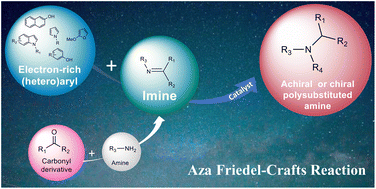
Nicolas Masurier was born in Yvetôt (France) in 1977. He first received his PharmD degree in 2003, before he obtained his PhD in 2006 from the University of Rouen (France) under the supervision of Prof. O. Lafont, working on the synthesis of β-cyclodextrin derivatives with hydrolytic activity against organophosphorous neurotoxics. Then, he moved to the University of Clermont-Ferrand (France) in the group of Pr. J.C. Teulade as an assistant-lecturer and worked on heterocyclic derivatives as tyrosine kinase inhibitors. He is currently professor in medicinal chemistry at the Faculty of Pharmacy of Montpellier. He worked at the Institut des Biomolécules Max Mousseron (IBMM), where his current research interests focus on the design, the preparation of new anticancer agents, PROTACs and kallikrein inhibitors.
Contact:
nicolas.masurier@umontpellier.fr
+33 (0)4 48 79 21 84
5 major publications :
S. Aït Amiri, C. Deboux, F. Soualmia, N. Chaaya, M. Louet, E. Duplus, S. Betuing, B. Nait Oumesmar, N. Masurier, C. El Amri, Identification of First-in-Class Inhibitors of Kallikrein-Related Peptidase 6 That Promote Oligodendrocyte Differentiation, Journal of Medicinal Chemistry, 2021, 64 (9), 5667-5688
C. Bonnel, B. Legrand, M. Simon, M. Clavié, A. Masnou, E. Jumas-Bilak, Y.K. Kang, P. Licznar-Fajardo, L.T. Maillard, L.T., N. Masurier, Tailoring the Physicochemical Properties of Antimicrobial Peptides onto a Thiazole-Based γ-Peptide Foldamer, Journal of Medicinal Chemistry, 2020, 63 (17), 9168-9180
A. Feral, G. Laconde, M. Amblard, N. Masurier, PROteolysis TArgetting Chimeras (PROTACs) strategy applied to kinases: recent advances, Advanced Therapeutics, 2020, 3 (11), 2000148
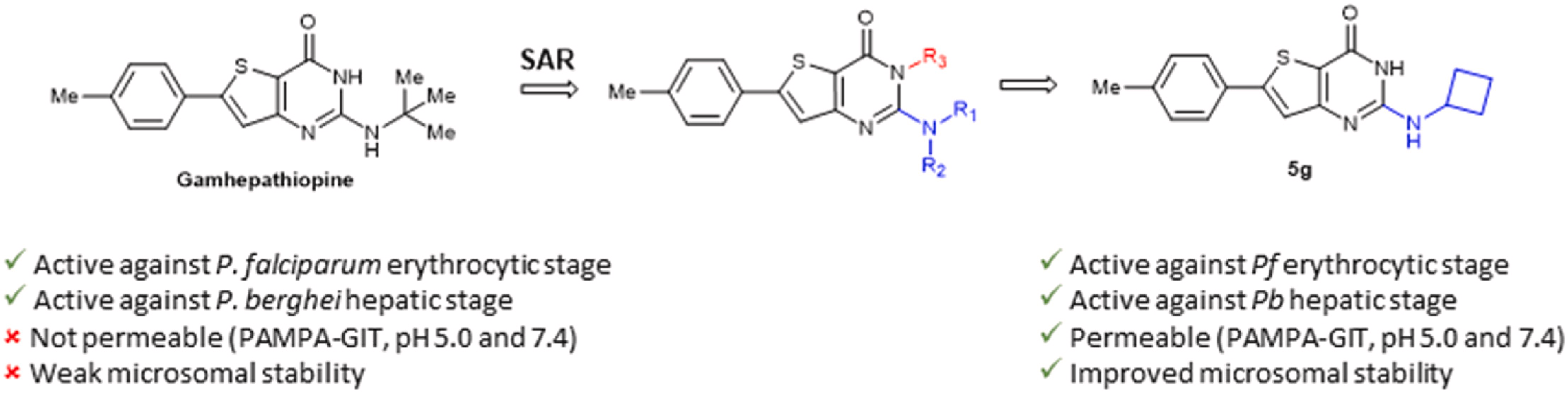
P. Le Baccon-Sollier, Y. Malki, M. Maye, L. M.A. Ali, L. Lichon, P. Cuq, L.-A. Vincent, N. Masurier, Imidazopyridine-fused [1,3]diazepinones: Modulations of positions 2 to 4 and their impacts on the anti-melanoma activity, Journal of Enzyme Inhibition and Medicinal Chemistry, 2020, 35 (1), 935-949
V. Bellet, L. Lichon, D. P. Arama, A. Gallud, V. Lisowski, L. T. Maillard, M. Garcia, J. Martinez, N. Masurier, Imidazopyridine-fused [1,3]-diazepinones Part 2: Structure-Activity Relationships and antiproliferative activity against melanoma cells, Eur. J. Med. Chem., 2017, 125, 1225-34