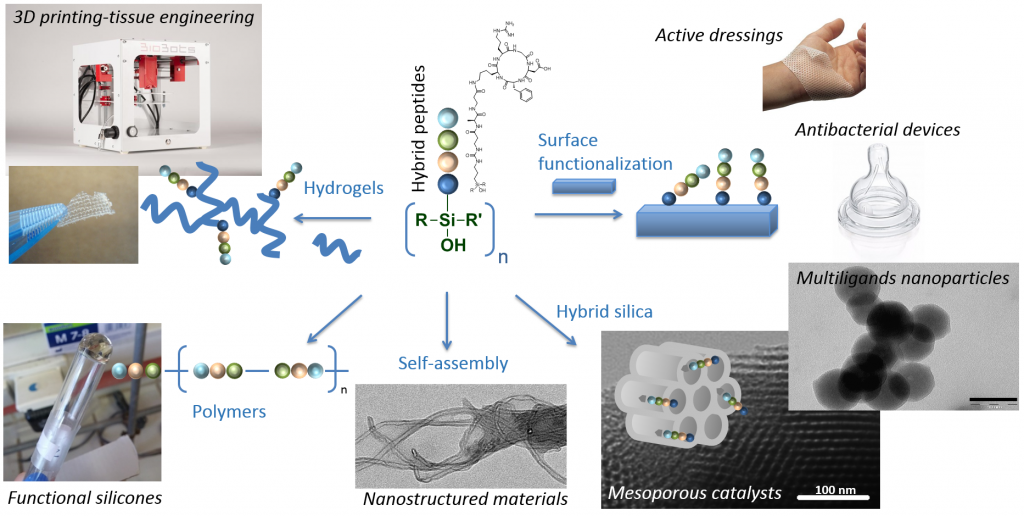
With their outstanding range of structures, structural and biological activities, peptides are highly attractive molecules to give a tailored function to an existing material but also to design innovative materials with unprecedented properties. Existing approaches to functionalise materials with biomolecules mostly relies on post-modification using conjugation chemistry (e.g. click reactions, activated esters). On the contrary, we envisioned innovative bottom-up approaches based on peptide building-blocks bearing functions for polymerisation or condensation. We investigated organic polymerisation using peptides bearing N-carboxyanhydride (NCA) moieties or lactame rings but also inorganic polymerisation methods, using sol-gel process relying on hydroxysilane-derivatized peptides.
Applications are numerous and some of them are currently investigated thought founded programs and collaborations :
• The functionalization of medical devices and dressing with wound-healing and/or antibacterial peptides and the design of smart ‘communicant’ dressings using RFID technology.
• The synthesis of multi-ligands nanoparticles for cancer treatment and imaging.
• The synthesis of polymers for cell targeting and vectorization.
• The design of biomimetic hydrogels for cell-based therapies that can be printed as 3D scaffolds.
From a fundamental point of view, the self-assembly of hybrid peptides is also studied for the design of new nanostructured materials.
APAPTIDE

Funding: Carnot Chimie Balard Institute
November 2019- November 2022
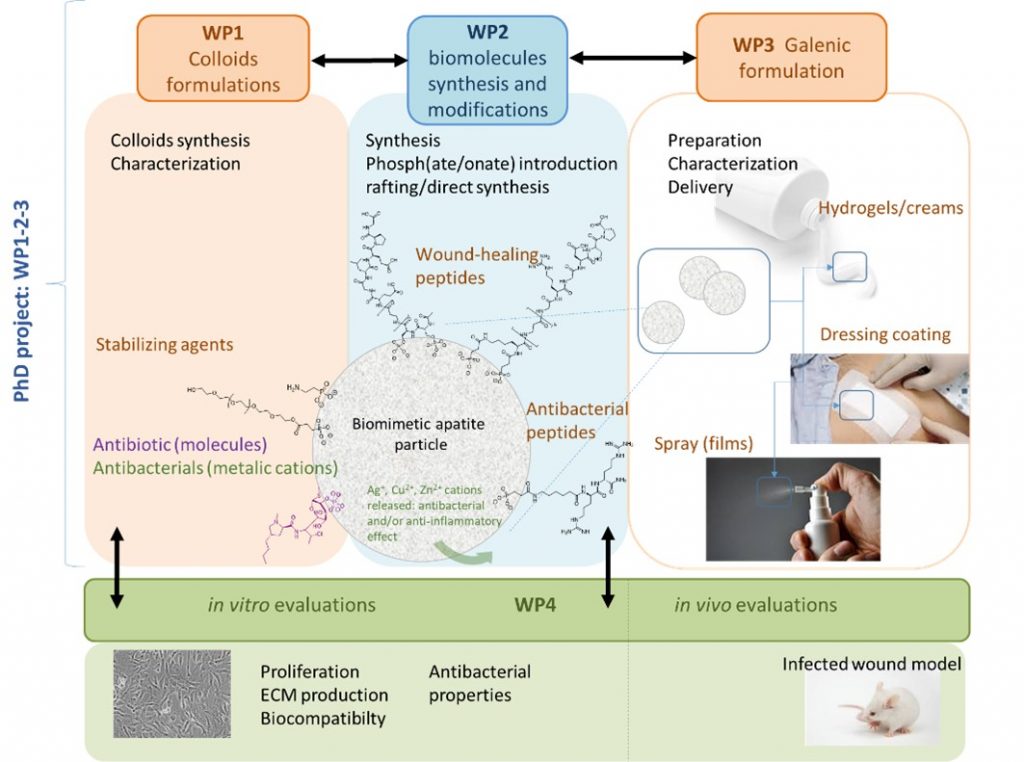
Open wounds are common in all health services, from emergencies to dermatology, maxillofacial, orthopedic and plastic surgery. In the case of deep wounds, infection is a major threat to the health of the patient, especially for the most fragile subjects such as the elderly, diabetic patients, immuno-depressed or burn victims. In addition, current solutions are generally based on general antibiotic therapy, but microbial resistance is becoming a major public health problem, and alternative solutions are needed.
In this context, APAPTIDE aims to design an alternative solution to conventional dressings and systemic antibiotic treatments through the development of sub-micron peptide-apatite hybrid particles for the controlled release of active agents. Such anti-inflammatory or antibacterial agents will be associated with apatitic particles by ion doping (ex: ions Cu2 +, Zn2 +), and placed on the organic corona stabilizing apatite particles in colloidal solution. In this project and for the first time, this organic corona will be constituted by bio active peptides displaying antimicrobial, pro-angiogenic (promoting neovascularization) and wound healing properties. The innovative aspect of the project is based on the very nature of the hybrid objects.
This project is developed in collaboration between IBMM (Montpellier) and CIRIMAT (Toulouse).
THE TEAM

Gilles Subra

MATHILDE GUÉRIN

CHRISTOPHE DROUET
PEPTIMPRINT

Funding: ANR-18-CE19-023-02
January 2019- June 2022
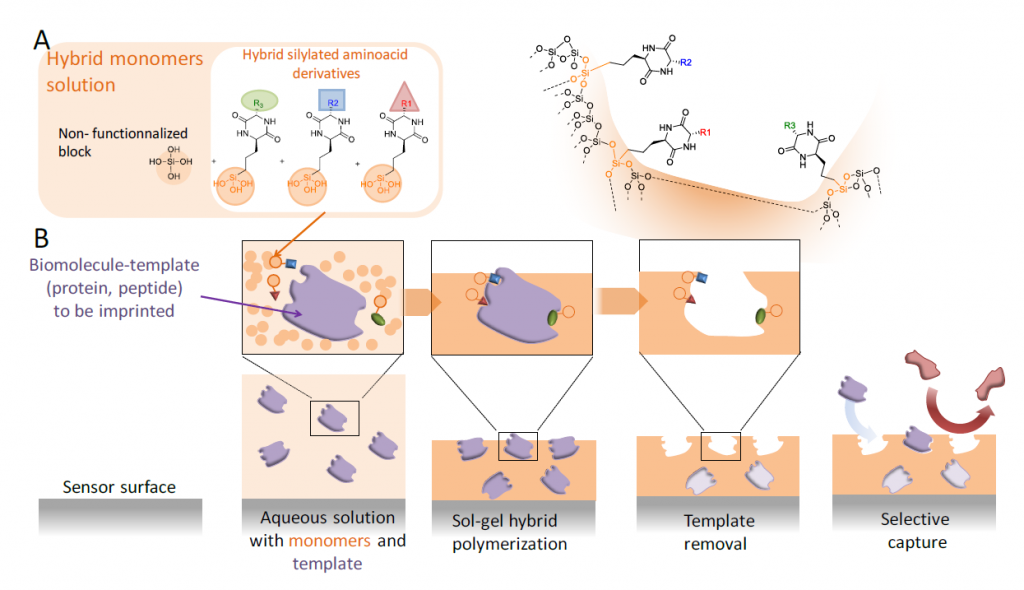
The concept of ‘molecular imprinting’ emerged in the 1930’s and thanks to its conceptual simplicity, it always attracted a tremendous interest of many scientific communities. The goal is to obtain the hollow shape of a molecule (i.e. ‘template ‘) in a three-dimensional matrix. The template is then removed from the material to give the empty cavities, which are now able to selectively capture the initial object (i.e. the template molecule). These molecular imprints were obtained either inside a material (e.g. hydrogel, macroporous matrix etc.) or on the surface of a substrate. Both organic or inorganic polymerization of monomeric precursors have been used to prepare imprinted materials. However, most of precursors (monomers) commonly used for molecular imprinting are organic ones that react between them by free radical polymerization.
In this sense, the objective of Peptimprint is to set-up a ground-breaking technology to prepare highly specific tridimensional and functionalized imprints of biomolecules (peptides, proteins) by template-assembly, but in this case following the inorganic polymerization by sol-gel process. For that, a variety of original hybrid building blocks mimicking amino acids (diketopiperazine) will be synthetised and developed to be used as precursors (functional monomers) in the MIP synthesis. Unfunctionalized blocks (e.g. tetraethoxysilane (TEOS), methyltriethoxysilane, dimethyldiethoxysilane, bis(triethoxysilyl) ethylene, mono-silylated PEG or bis-silylated PEG) will be also used concomitantly to create the network. Once the template molecule removed, the hollow cavities will be able to capture the biomolecule which was previously imprinted with very high selectivity and affinity. Indeed, unlike classical molecular imprint polymers (MIPs), the resulting inorganic/bioorganic hybrid material will recapitulate not only the shape but also the complementary functions of the biomolecular template.
This project is developed in collaboration with team “Analytic Sciences of Biomolecules & Molecular Modelling” of the Institute of Biomolecules Max Mousseron (IBMM), the team “Molecular Chemistry and Solid-state Organization (CMOS)” of the Institute Charles Gerhardt (ICG), the team “Optomicrofluidique (POMM)” from the Charles Coulomb laboratory of the University of Montpellier, and the team interactions of the “Institut de Recherche en Cancérologie de Montpellier” (IRCM).
THE TEAM

Gilles Subra

AHMAD MEHDI

CATHERINE PERRIN

YOANN LADNER

PASCAL VERDIÉ

PASCAL ETIENNE

PIERRE MARTINEAU

MARTINE PUGNIÈRE

RAQUEL GUTIÉRREZ-CLIMENTE

JÉRÉMIE GOUYON

GIANG NGO

MARGAUX CLAVIÉ
Sol-gel polymerization of silylated amino acids around a protein template yields selective biomimetic imprints
Raquel Gutiérrez-Climente, Giang Ngo, Margaux Clavié, Jérémie Gouyon, Yoann Ladner, Pascal Etienne, Pascal Dumy, Catherine Perrin, Ahmad Mehdi, Pierre Martineau, Martine Pugnière and Gilles Subra
Abstract
The development of artificial receptors able to selectively recognize a target protein is of particular interest in separation, diagnostics, and therapeutics fields. Herein, we disclose a method to prepare biomimetic and functionalized protein imprints in biocompatible conditions avoiding any protein denaturation. For that purpose, a set of different hybrid silylated amino acid derivatives were synthesized and used without tetraethyl orthosilicate to prepare our molecularly imprinted polymers, allowing to reduce to a minimum of the silicon amount, in order to obtain imprints made almost entirely of amino acids to mimic paratope surfaces of antibodies. Such functional building blocks were polymerized on the surface of magnetic silica nanoparticles at pH 8.5 in ultrapure water in the presence of two globular proteins: cytochrome C or lysozyme. The resulting imprinted hybrid materials were evaluated for their adsorption capacity, specificity, and selectivity by quartz-crystal microbalance with dissipation and magnetic enzyme-linked immunosorbent assay (ELISA) assays. High imprinting factors of 8.7 were measured for these biomimetic hybrid materials (corresponding to approximately 4000 and 450 ng of protein per cm2 immobilized on molecularly imprinted polymers and non-imprinted polymer nanoparticles, respectively), representing a significant breakthrough in sol-gel-based molecular imprinting materials. Moreover, competition experiments performed by magnetic ELISA (mELISA) show very good specificity of our imprints at the usual concentrations of ELISA measurements.
Development of Amino Acids Functionalized SBA-15 for the Improvement of Protein Adsorption
Molecules 2021, 26(19), 6085; https://doi.org/10.3390/molecules26196085
Raquel Gutiérrez-Climente, Margaux Clavié, Jérémie Gouyon, Giang Ngo, Yoann Ladner, Pascal Etienne, Pascal Dumy, Pierre Martineau, Martine Pugnière, Catherine Perrin, Gilles Subra and Ahmad Mehdi
Abstract
Ordered mesoporous materials and their modification with multiple functional groups are of wide scientific interest for many applications involving interaction with biological systems and biomolecules (e.g., catalysis, separation, sensor design, nano-science or drug delivery). In particular, the immobilization of enzymes onto solid supports is highly attractive for industry and synthetic chemistry, as it allows the development of stable and cheap biocatalysts. In this context, we developed novel silylated amino acid derivatives (Si-AA-NH2) that have been immobilized onto SBA-15 materials in biocompatible conditions avoiding the use of toxic catalyst, solvents or reagents. The resulting amino acid-functionalized materials (SBA-15@AA) were characterized by XRD, TGA, EA, Zeta potential, nitrogen sorption and FT-IR. Differences of the physical properties (e.g., charges) were observed while the structural ones remained unchanged. The adsorption of the enzyme lysozyme (Lyz) onto the resulting functionalized SBA-15@AA materials was evaluated at different pHs. The presence of different functional groups compared with bare SBA-15 showed better adsorption results, for example, 79.6 nmol of Lyz adsorbed per m2 of SBA-15@Tyr compared with the 44.9 nmol/m2 of the bare SBA-15.
Sol-gel process – the inorganic approach in protein imprinting
J. Mater. Chem. B, 2021 Jan. https://doi.org/10.1016/j.xphs.2020.10.019
Raquel Gutiérrez-Climente, Margaux Clavié, Pascal Dumy, Ahmad Mehdi and Gilles Subra
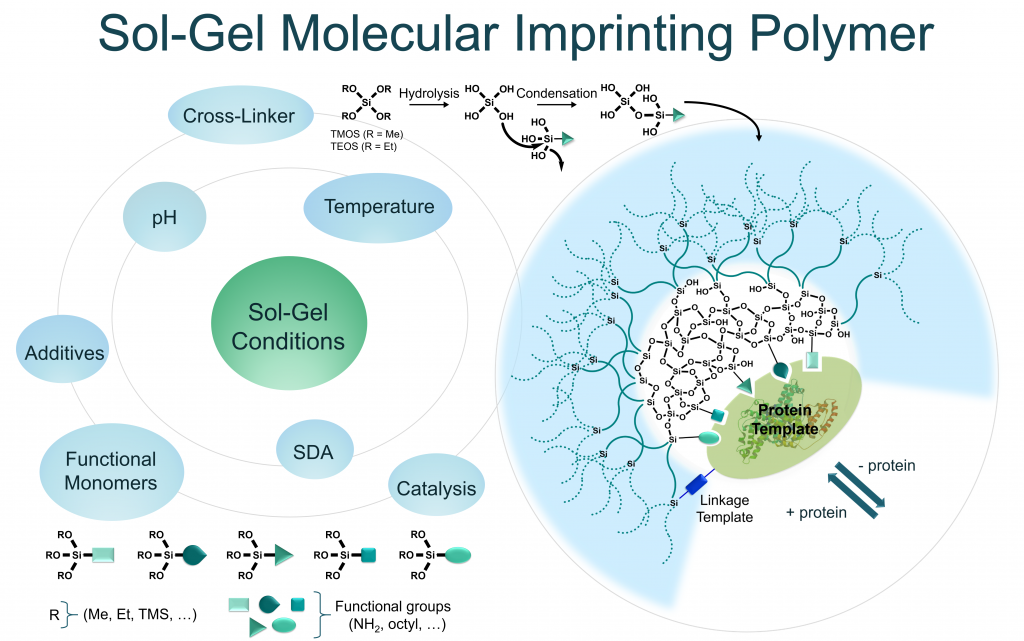
Abstract
Proteins play a central role for the signal transmission in living systems since they are able to recognize specific biomolecules acting as cellular receptors, antibodies or enzymes; or being themselves recognized by other proteins in protein/protein interactions, or displaying epitopes suitable for antibody binding. In this context, the specific recognition of a given protein, unlocks a range of interesting applications in diagnosis and in targeted therapies. Obviously, this role is already fulfilled with antibodies with unquestionable success. However, the design of synthetic artificial systems able to endorse this role is still challenging with a special interest to overcome limitations of antibodies, in particular their production and their stability. Molecular Imprinted Polymers (MIPs) are attractive recognition systems which could be an alternative for the specific capture of proteins in complex biological fluids. MIPs can be considered as biomimetic receptors or antibodies mimics displaying artificial paratopes. However, MIPs of proteins remains a challenge due to their large size and conformational flexibility, their complex chemical nature with multiple recognition sites and their low solubility in most organic solvents. Classical MIP synthesis conditions result in large polymeric cavities with and unspecific binding sites on the surface. In this review, the potentiality of sol-gel process as inorganic polymerization strategy to overcome the drawbacks of protein imprinting is highlighted. Thanks to the mild and biocompatible experimental conditions required and the use of water as solvent, the inorganic polymerization approach suits better to proteins than organic polymerization. Through numerous examples and applications of MIPs, we proposed a critical evaluation of the parameters that must be carefully controlled to achieve sol-gel protein imprinting (SGPI), including the choice of the monomers taking part in the polymerization.
| Date | Congress | Type presentation | Title presentation |
| 15-18 June 2021 | Balard Chemistry Conferences 2021 | Oral Communication | Biomimetic protein imprinting on magnetic particles using amino acid-based hybrid blocks |
| 18 June 2021 | aesap (Association des Enseignants de Sciences Analytiques de Pharmacie | Flash Communication | Silylated amino acids as hybrid precursors for protein-biomimetic surface coating: application to electrophoresis separation |
| 22-24 June 2021 | Affinity 2021 | Flash Communication | Amino acid-based hybrid blocks for biomimetic protein imprinting |
| 5-7 July 2021 | ISOS-2021 The 19th International Symposium on Silicon Chemistry | Flash Communication | |
| 14 July 2021 | e-MSB 2021 Boston | Oral Communication | Silylated amino acids as hybrid precursors for protein-biomimetic surface coating: application to electrophoresis separation |
| 5-7 October 2021 | SEP21- 14ème Congrès Francophone sur les Sciences Séparatives et les Couplages de l’AFSEP | Oral communication | Silylated amino acids as hybrid precursors for protein-biomimetic surface coating: application to electrophoresis separation
|
| 28-29 October 2021 | JMJC2021 9èmes Journées Méditerranéennes des Jeunes Chercheur(e)s | Poster Communication | Applications of silylated amino acid in the development of hybrid materials |
| Oral Communication | Silylated amino acids as hybrid precursors for molecularly imprinted polymers |
||
| 2-3 November 2021 | QSense User Days – Biolin Scientific | Oral Communication | Using QCM-D to study the recognition of molecularly imprinted polymer particles toward their target proteins |
ORALCANCERPRINT
Biofabrication of an oral squamous cell carcinoma model by 3D bioprinting

Funding : Emergence, Cancéropôle Grand Sud-Ouest
June 2018 – June 2019
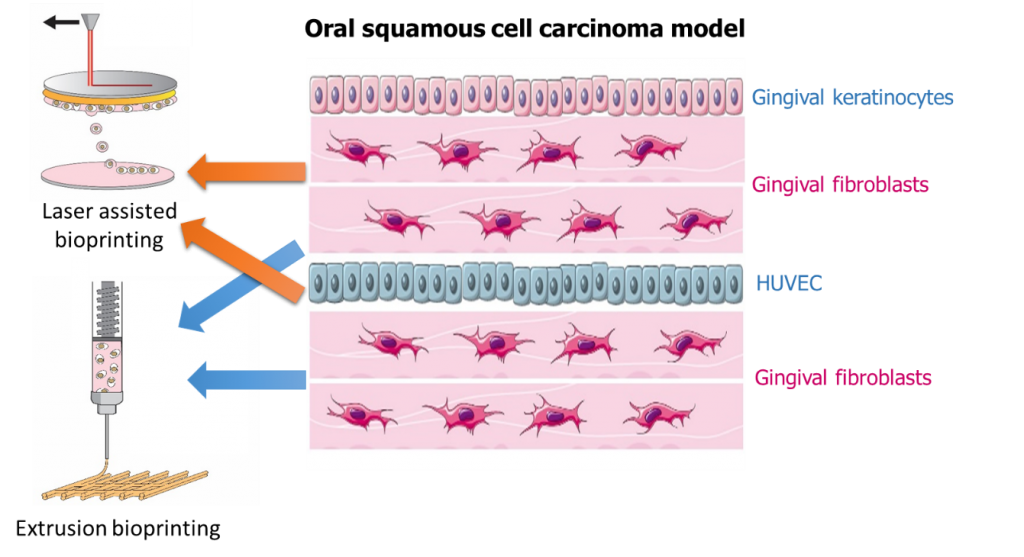
Nowadays, treatments of oral squamous cell carcinoma is principally based on excision surgery and can be associated with radiotherapy and chemotherapy. It is really important to have a better understanding of the neoplastic oral mucous membrane to develop less aggressive treatment. This project is a multidisciplinary project aiming at designing an oral squamous cell carcinoma model by 3D bioprinting, to help the development of cancer treatments.
The aim of the project is to develop an in vitro 3D organotipic model of oral mucous membrane with the help of 3D-bioprinting of cellularized layers. The use of two different 3D bioprinting technologies will allow to reproduce the architecture of cancer tissues at a macroscopic and a microscopic scale. Layers of hybrid collagenous peptide based hydrogel encapsulating gingival fibroblasts will be 3D printed by extrusion with layers of endothelial cells in the same hydrogel but 3D printed by a laser-assisted technology, to create a vascularized tissue. Finally, a layer of cancerous keratinocytes embedded into the hydrogel, printed by laser, will cover the model. The complexity of the model will give a better understanding of cellular communication in oral cancer and will be a platform for drug screening.
This project is developed in collaboration with BioTis Team ( Dr. Adrien Naveau and Dr. Raphael Devillar), Inserm 1026 : Tissular Bio-engineering team, Bordeaux.
THE TEAM

Gilles Subra

RAPHAEL DEVILLAR (BioTis)

ADRIEN NAVEAU (BioTis)

AHMAD MEHDI

LAURINE VALOT
E-dressing
The e-dressing project aims at developing a smart wound dressing able to detect an enzymatic activity and to transmit information by passive RFID (radio frequency identification)

Funding: MUSE research founding
Mar 2018- Feb 2021
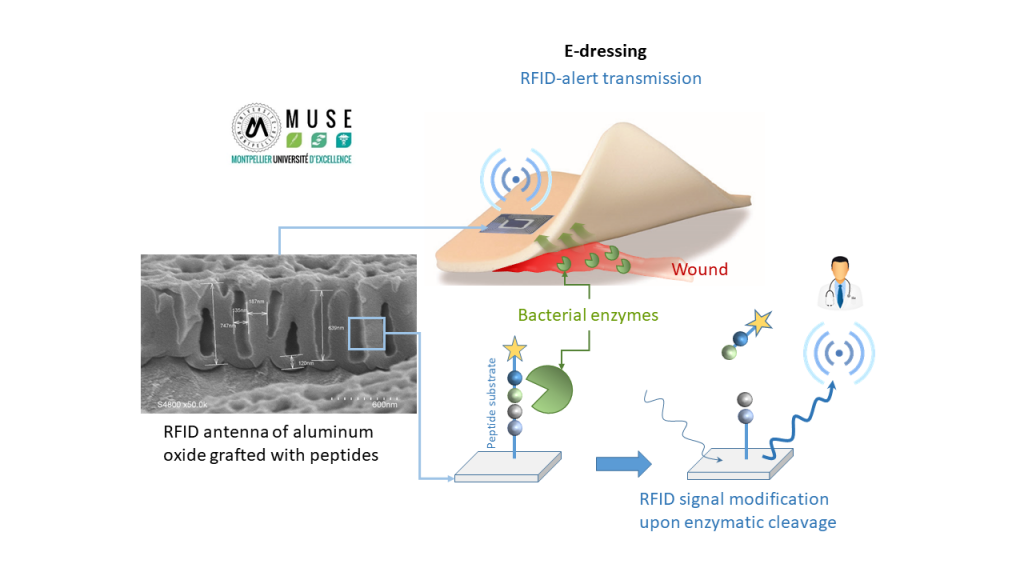
E-dressing project
The e-dressing project aims at developing a smart wound dressing able to detect an enzymatic activity and to transmit information by passive RFID (radio frequency identification).
The cost of ineffective treatment of chronic wounds (diabetic foot, leg ulcers…) is estimated to be $20-25 billion annually. Efficient and simple wound diagnostic devices could reduce the complications associated with infection or excessive inflammation being able to target the right therapy, at the right time.
We chose to focus on the design of the RFID device which will be included into the dressing, in contact with the wound exudates. The antenna of RFID device, constituted of porous aluminum oxide, will be grafted by a layer of hybrid material, sensitive to the degradation by bacterial enzymes, virulence markers, or by enzymes related to the inflammation of wounds.
Hybrid material will be composed of hybrid silylated peptides whose sequences will be chosen to be enzyme substrates. Material will be synthesized by sol-gel process and will be degraded specifically by these chosen enzymes.
The first part of the work consists in the design and synthesis of hybrid peptides that will be modified by alkoxysilane groups. They will allow their immobilization by sol-gel process on aluminium oxide substrates.
The second part of the work aims the characterization of the hybrid material (AFM, XPS, SEM…) layer during its degradation. In vitro assays in the presence of isolated enzymes and with relevant bacterial strains will be performed.
The aim of the third part of project is to demonstrate that the degradation of hybrid material by enzymes will generate a detectable modification of electrical signal. Indeed, this electrical variation will impact on the RFID reading and the putative quantification of the targeted enzymes.
At last, the system will be assayed on infected exsudates of patients, collected at Montpellier University Hospital.
E-dressing is multidisciplinary project supported by Montpellier University Site of Excellence which also involves the Institute of Electronics and Systems (IES-team Brice Sorli), the University Hospital of Montpellier (CHU-teams Eric Renard and Luc Teot), Institute Charles Gerhart of Montpellier (ICG-team Ahmad Mehdi) and the Ecole des Mines d’Ales (EAM, team Ingrid Bazin)
THE TEAM

Gilles Subra

AHMAD MEHDI

AMANDINE SANDOVAL
LEGOGEL
A 'Lego'-like method to access multifunctional hydrogels for mesenchymal stem cell-based cartilage repair

Funding: ANR-16-CE18-0003-01
October 2016- September 2019
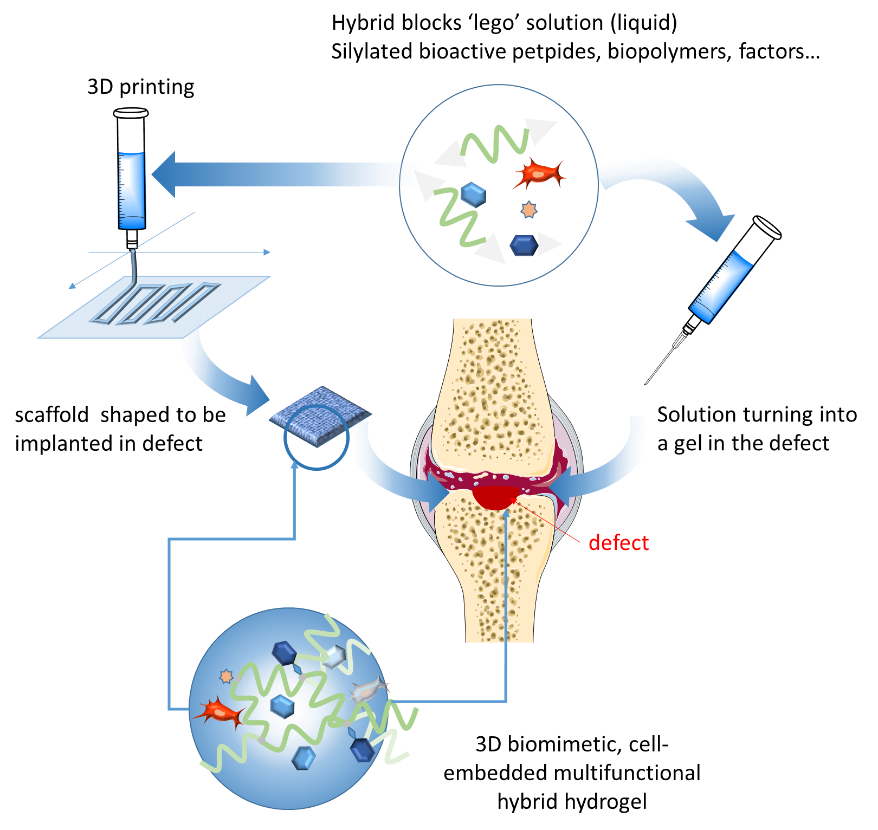
The LEGOGEL multidisciplinary project combines peptide chemistry and sol-gel inorganic polymerization to provide multifunctional hydrogels for full-thickness cartilage lesion repair using mesenchymal stem cells (MSC).
Treatment of cartilage injuries remains one of the most difficult challenges in regenerative medicine. Mesenchymal stem cell (MSC)-based therapies represent one possible innovative strategy. It is highly important that MSC will be associated with a support, to put the cells directly into the lesion to be repaired but also to avoid dissemination at the time of implantation. The support can be a scaffold shaped to fit the cartilage defect or a liquid that can be injected and can rapidly turn into a gel to fill the lesion. LEGOGEL is a multidisciplinary project aiming at designing cell therapy strategies for osteo-articular diseases.
Legogel Project
LEGOGEL aims at establishing a ground-breaking method to build modular and multifunctional hydrogels whose synthesis was too complex to be envisioned by existing methods. LEGOGEL relies on original ‘hybrid’ bioorganic-inorganic building blocks (peptides, biopolymers, dyes…) bearing alcoxysilane groups. Hydrolysis and condensation of these groups lead to the formation of a covalent hydrogel network. Interestingly, this sol-gel process occurs in water in physiological conditions, without the use of chemical cross-linkers, toxic reagents or catalysts. It is thus compatible with biomolecules (ligands, growth factors…) and the presence of cells. Moreover, the mixture of blocks is first obtained as a colloidal solution that can be either injected, or 3D-printed before complete gelation to get porous scaffolds. Taking advantage of this innovative ‘lego-like’ bottom-up approach, the complexity of extracellular matrices (ECM) composed of a huge variety of biological components, could be mimicked. Any type of bioactive peptide, biopolymer, dye or contrast agent can be combined in appropriate ratio to yield covalent hydrogel. Of course, any other component (as growth factors) can be added during the gel formation, in a non-covalent way.
Specific objectives of LEGOGEL project
- The first goal will be the synthesis of the different hybrid blocks (i.e. the ‘lego parts’). Bioactive peptide promoting cell migration and colonization, biopolymers (growth factors) and contrast agents will be specifically silylated. The synthesis of hybrid bioactive peptides is already mastered by the partners but the introduction of silyl groups on proteins is a challenge that will be tackled.
- The second objective will be to obtain multifunctional hydrogels whose biological and physicochemical properties (stiffness, porosity, degradability…) will be suitable for cell-based engineering. The obtainment of hydrogel substrates from liquid solution will be studied. The most challenging work will be the control of gelation conditions to transfer the soft polymerization method on the 3D printer for the biofabrication of cell-seeded porous scaffolds. If required, Peptide sequences sensitives to ECM degradation enzymes will be incorporated to tune the degradation rate of the matrix and to release the differentiation factors to induce the lineage commitment.

3D printer used for the project - The third objective will be to obtain optimized hydrogel substrates promoting in vitro and in vivo MSC colonization and differentiation into chondrocytes or osteoblasts. Different composition of hydrogels, containing differentiation factors tailored to mimic specific cartilage and subchondral bone environments will be assayed to obtain the ‘ideal’ substrate. It will be either 3D-printed as a scaffold or used as an injectable hydrogel. Ultimately, the scaffold will present a bilayer membrane composed of TGFβ3- hyaluronic acid upper layer to induce differentiation of MSCs into chondrocytes and secretion of the cartilage ECM; and BMP2-hydroxyapatite-collagen lower layer to favor the differentiation of MSC into osteoblasts.
THE TEAM

Gilles Subra

AHMAD MEHDI

LAURINE VALOT

MARIE MAUMUS

LUC BRUNEL

DANIÈLE NOËL

PASCAL VERDIÉ
CONTACT
Simple and Specific Grafting of Antibacterial Peptides on Silicone Catheters
Advanced Healthcare Materials 5: 3067–3073, 2016. https://doi.org/10.1002/adhm.201600757
Abstract
To fight against nosocomial infection initiated by colonization of medical devices, a strategy enabling the direct and fast functionalization of silicone surfaces is proposed. This strategy proceeds in a site-specific way using original hybrid silylated antibacterial peptides. This safe and up-scalable method guarantees a covalent and robust immobilization with the correct orientation of the bioactive moiety. Importantly it also avoids multi-step chemical modifications of the surface or multi-layer polymer coatings. As proof of concept, antibacterial silicone catheter has been prepared whose immediate and long term efficiency is superior by comparison to similar silver-embedded materials.
Direct Synthesis of Peptide-Containing Silicones: A New Way to Bioactive Materials
Chemistry A European Journal, 26:12839-12845, 2020. https://doi.org/10.1002/chem.202001571
Abstract
A simple and efficient way to synthesize peptide-containing silicone materials is described. Silicone oils containing a chosen ratio of bioactive peptide sequences were prepared by acid-catalyzed copolymerization of dichlorodimethylsilane, hybrid dichloromethyl peptidosilane, and Si(vinyl)- or SiH-functionalized monomers. Functionalized silicone oils were first obtained and then, after hydrosilylation cross-linking, bioactive polydimethylsiloxane (PDMS)-based materials were straightforwardly obtained. The introduction of an antibacterial peptide yielded PDMS materials showing activity against Staphylococcus aureus. PDMS containing RGD ligands showed improved cell-adhesion properties. This generic method was fully compatible with the stability of peptides and thus opened the way to the synthesis of a wide range of biologically active silicones.
Silylated biomolecules: Versatile components for bioinks
Abstract
Physical hydrogels prepared from natural biopolymers are the most popular components for bioinks. However, to improve the mechanical properties of the network, in particular its durability for long-lasting tissue engineering applications or its stiffness for bone/cartilage applications, covalent chemical hydrogels have to be considered. For that purpose, biorthogonal reactions are required to allow the inclusion of living cells within the bioink reservoir before the 3D printing procedure. Interestingly, such reactions also unlock the possibility to further multifunctionalize the network, adding bioactive moieties to tune the biological properties of the resulting printed biomaterial. Surprisingly, compared to the huge number of studies disclosing novel bioink compositions, no extensive efforts have been made by the scientific community to develop new chemical reactions meeting the requirements of both cell encapsulation, chemical orthogonality and versatile enough to be applied to a wide range of molecular components, including fragile biomolecules. That could be explained by the domination of acrylate photocrosslinking in the bioprinting field. On the other hand, proceeding chemoselectively and allowing the polymerization of any type of silylated molecules, the sol-gel inorganic polymerization was used as a crosslinking reaction to prepare hydrogels. Recent development of this strategy includes the optimization of biocompatible catalytic conditions and the silylation of highly attractive biomolecules such as amino acids, bioactive peptides, proteins and oligosaccharides. When one combines the simplicity and the versatility of the process, with the ease of functionalization of any type of relevant silylated molecules that can be combined in an infinite manner, it was obvious that a family of bioinks could emerge quickly. This review presents the sol-gel process in biocompatible conditions and the various classes of relevant silylated molecules that can be used as bioink components. The preparation of hydrogels and the kinetic considerations of the sol-gel chemistry which at least allowed cell encapsulation and extrusion-based bioprinting are discussed.
3D high-precision melt electro written polycaprolactone modified with yeast derived peptides for wound healing
Abstract
In this study melt electro written (MEW) scaffolds of poly(ε-caprolactone) PCL are decorated with anti-inflammatory yeast-derived peptide for skin wound healing. Initially, 13 different yeast-derived peptides were screened and analyzed using both in vitro and in vivo assays.
The MEW scaffolds are functionalized with the selected peptide VLSTSFPPW (VW-9) with the highest activity in reducing pro-inflammatory cytokines and stimulating fibroblast proliferation, migration, and collagen production. The peptide was conjugated to the MEW scaffolds using carbodiimide (CDI) and thiol chemistry, with and without plasma treatment, as well as by directly mixing the peptide with the polymer before printing. The MEW scaffolds modified using CDI and thiol chemistry with plasma treatment showed improved fibroblast and macrophage penetration and adhesion, as well as increased cell proliferation and superior anti-inflammatory properties, compared to the other groups. When applied to full-thickness excisional wounds in rats, the peptide-modified MEW scaffold significantly enhanced the healing process compared to controls (p < 0.05). This study provides proof of concept for using yeast-derived peptides to functionalize biomaterials for skin wound healing.
One-Pot Synthesis of Bioinspired Peptide-Decorated Apatite Nanoparticles for Nanomedicine
Abstract
Hybrid organic-inorganic bio-inspired apatite nanoparticles (NPs) are attractive for biomedical applications and especially in nanomedicine. Unfortunately, functionalized apatite NPs were so far synthesized by multistep processes and often yielding broad particle size distributions. In addition, although the adsorption of drugs or antibodies on apatite NPs surfaces was reported, it was generally associated with uncontrolled drug loading. All these limitations have restricted their actual applications in nanomedicine. Besides, very few attempts at exposing bioactive peptides on apatite NPs have been made. In this work, we report an original one-pot synthesis of well-defined bioactive hybrid NPs composed of a mineral core of bioinspired apatite surrounded by an organic corona of bioactive peptides. Dual stabilizing-bioactive agents, phosphonated PEG-peptide conjugates, were prepared and directly used during apatite precipitation (i) to form the organic corona during apatite precipitation, driving the size and shape of resulting hybrid NPs with colloidal stabilization and (ii) to expose peptide moieties (RGD or YIGSR sequences) at the NPs periphery in view of conferring additional surface properties to enhance their interaction with cells. Here, we demonstrate the success of this approach, we fully characterized the functionalized NPs by FTIR, Raman, XRD, solid and liquid state NMR, TEM and DLS, and we followed their interaction with fibroblast cells, unveiling a synergistic proliferative effect.
Hydrocarbon-Stapled Peptide Based-Nanoparticles for siRNA Delivery.
Nanomaterials (Basel). 2020 Nov 25;10(12):2334. doi: 10.3390/nano10122334
Simon M, Laroui N, Heyraud M, Laconde G, Ali LMA, Bourbiaux K, Subra G, Vezenkov LL, Legrand B, Amblard M, Bettache N.
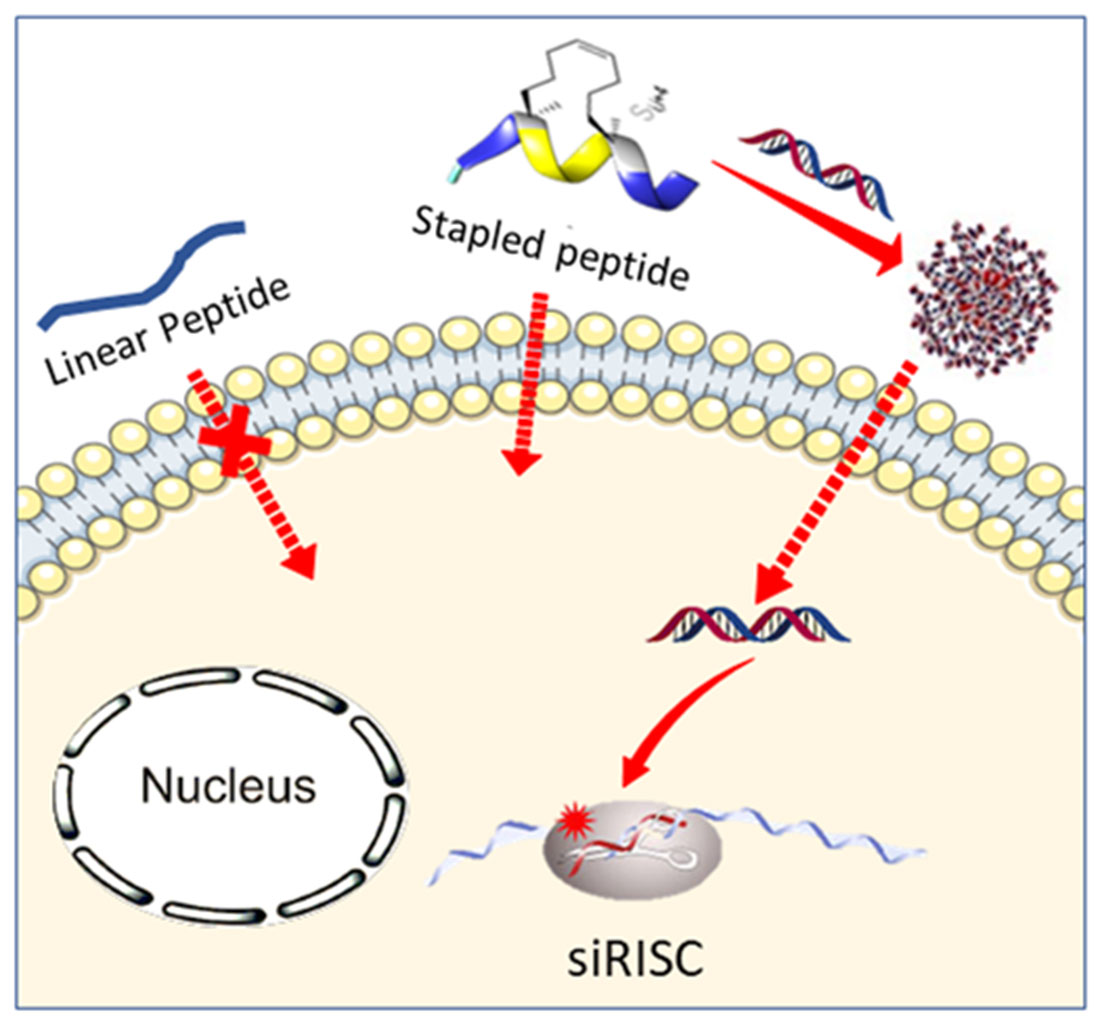
Abstract
Small interfering RNAs (siRNAs) are promising molecules for developing new therapies based on gene silencing; however, their delivery into cells remains an issue. In this study, we took advantage of stapled peptide technology that has emerged as a valuable strategy to render natural peptides more structured, resistant to protease degradation and more bioavailable, to develop short carriers for siRNA delivery. From the pool of stapled peptides that we have designed and synthesized, we identified non-toxic vectors that were able to efficiently encapsulate siRNA, transport them into the cell and induce gene silencing. Remarkably, the most efficient stapled peptide (JMV6582), is composed of only eight amino-acids and contains only two cationic charges.
Nano-assemblies with core-forming hydrophobic polypeptide via polymerization-induced self-assembly (PISA)
Polym. Chem., 2021,12, 113-121 DOI: 10.1002/macp.202000311
Dao T, Vezenkov L, Subra G, Ladmiral V, Semsarilar M.
Abstract
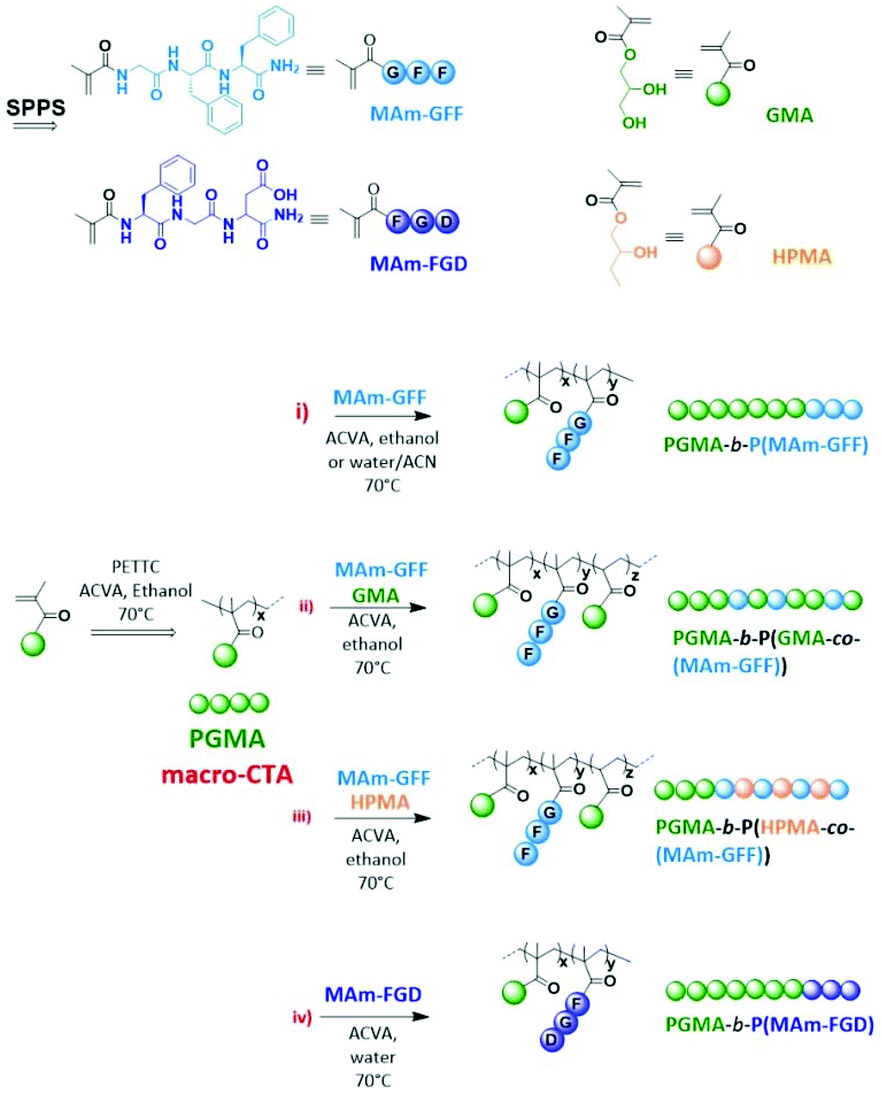
The aim of this study is to produce self-assembled structures with hydrophobic polypeptide cores via Reversible Addition–Fragmentation chain Transfer (RAFT) – mediated Polymerisation-Induced Self-Assembly (PISA). Hydrophilic poly(glycerol monomethacrylate) macromolecular chain transfer agents (PGMA mCTAs) were used to polymerize the self-assembling peptide monomers, resulting in the formation of diblock copolymer nano objects. Methacrylamide derivatives containing self-assembling tripeptides MAm-GFF (MAm-Gly-Phe-Phe-NH2) and MAm-FGD (MAm-Phe-Gly-Asp-NH2) were used as hydrophobic monomers. The self-assembling behaviours of these monomers mainly derive from the interactions of the phenylalanine residues, however their difference in hydrophobicity required different polymerization conditions. MAm-GFF was polymerized in the presence of organic solvent (ethanol or acetonitrile), under either dispersion or emulsion polymerization, while MAm-FGD was polymerized under aqueous dispersion conditions. PGMA-b-P(MAm-FGD) obtained from aqueous PISA typically formed fibrous structures while a range of morphologies such as fibre-, flake-, and leaf-like or spherical vesicles were obtained for PGMA-b-P(MAm-GFF) depending on the copolymer composition and solvent used. In all cases the peptides self-assembling core had a crucial influence on the final morphologies.
Bottom-up strategies for the synthesis of peptide-based polymers
Progress in Polymer Science 115 (2021) 101377 https://doi.org/10.1016/j.progpolymsci.2021.101377
Martin J, Desfoux A, Martinez J, Amblard M, Mehdi A, Vezenkov L, Subra G
Abstract
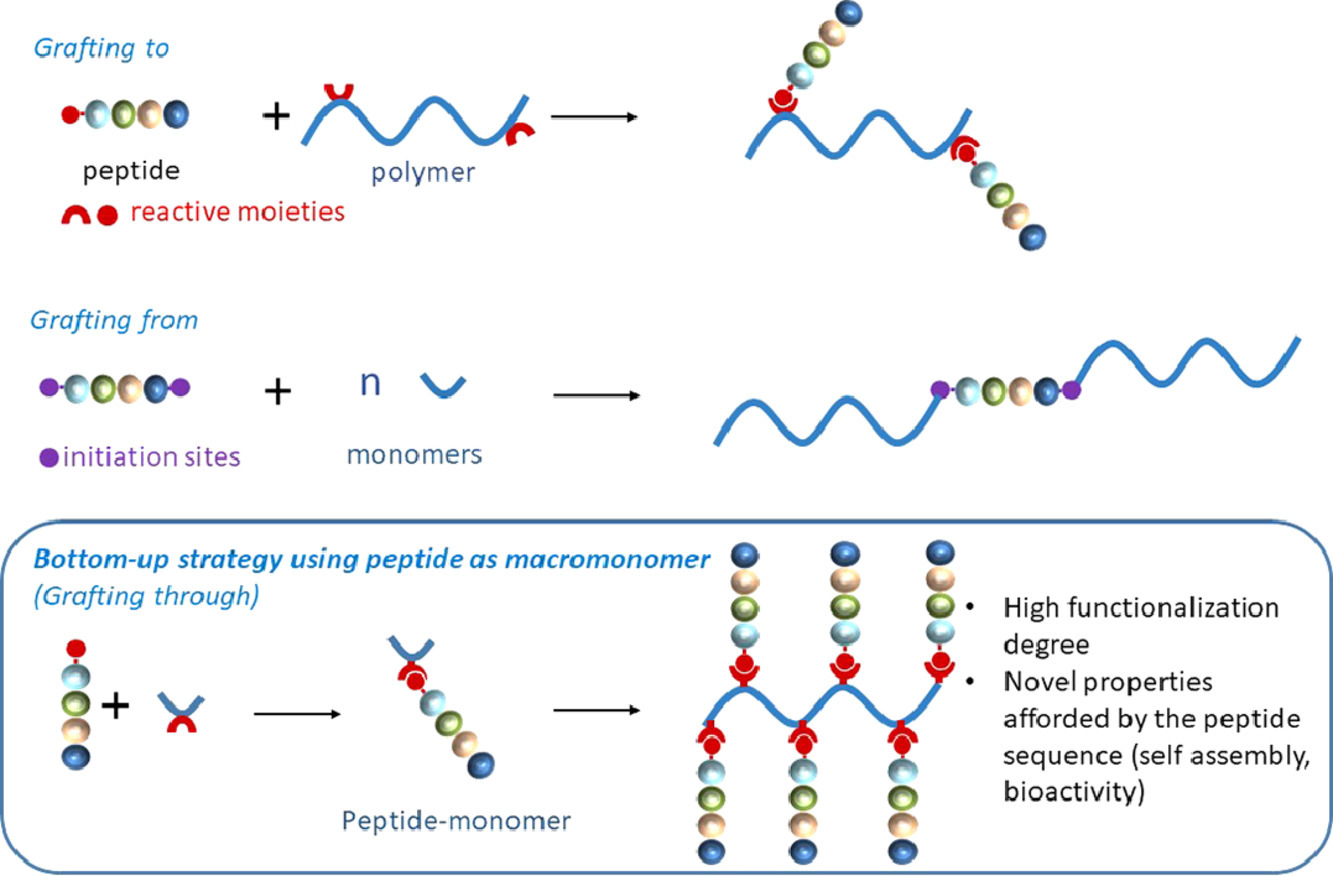
Thanks to their wide range of biological activities, peptides have been extensively used to afford designed materials with tailored properties. Peptides can be associated to polymers combining the properties of various polymer backbones with those of bioactive peptide sequences. Such conjugates find promising applications in medical devices, tissue engineering, drug targeting and delivery. Improvement of existing polymers by post-modification peptide grafting is achieved through an extensive range of organic reactions, involving the prior preparation of functional polymers displaying suitable anchoring functions. Alternatively, peptides can be used as initiators of polymerization yielding a chimeric molecule bearing a single peptide at the end of macromolecular chains. Finally, novel polymer materials can be designed when the peptide itself is used as a macromonomer. In that case, the unmatched level of repetition of the peptide sequence or/and its self-assembly properties allow to access very high functionalization degree, original structures and bioactivities.
Self-Assembling Peptide—Polymer Nano-Objects via Polymerization-Induced Self-Assembly
Macromolecules 2020, 53, 16, 7034–7043 https://doi.org/10.1021/acs.macromol.0c01260
Dao T, Vezenkov L, Subra G, Amblard M, In M, Le Meins J-F, Aubrit F, Moradi M-A, Ladmiral A, Semsarilar M*
Abstract
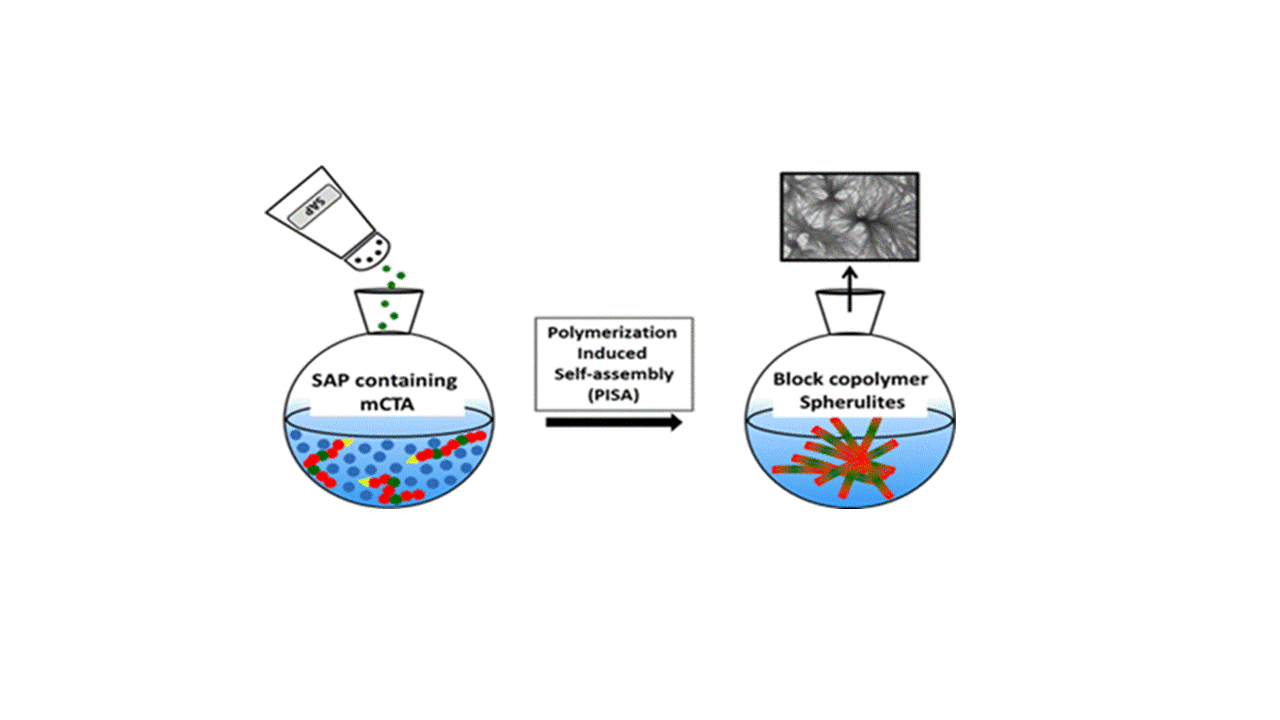
Self-assembling peptides (SAPs) have been extensively studied for their ability to form nanoscale ordered structures driven by noncovalent molecular interactions. Meanwhile, polymerization-induced self-assembly (PISA) has been exploited as a facile and efficient way to produce various amphiphilic block copolymer nano-objects, whose self-assembly was governed predominantly by the interactions of the different blocks with the polymerization medium. In this work, we combined PISA with SAPs to prepare novel peptide–polymer hybrid nano-objects, thus harnessing the advantages of PISA and the self-assembling driving force of SAPs. A tripeptide methacrylamide derivative (MAm-Gly-Phe-Phe-NH2, denoted as MAm-GFF, where MAm means methacrylamide) was copolymerized with glycerol monomethacrylate (GMA) to produce a P(GMA65–stat-(MAm-GFF)7) macro-chain transfer agent (macro-CTA) by reversible addition–fragmentation chain transfer polymerization in dimethylformamide. This peptide-based macro-CTA was then successfully chain-extended with poly(2-hydroxypropyl methacrylate) (PHPMA) by aqueous dispersion PISA, forming P(GMA65–stat-(MAm-GFF)7)-b-PHPMA28 self-assembled objects. Fibrous structures were observed by transmission electron microscopy (TEM) and Cryo-TEM, in agreement with depolarized dynamic light scattering, static light scattering, and small-angle X-ray scattering experiments that also revealed long anisotropic morphologies. Such structures have not been reported previously for PISA-prepared nano-objects. This confirms the decisive influence of the GFF SAP on the self-assembly. In addition, annealing the PISA suspension at different temperatures led to a significant size decrease in the self-assembled objects and to a morphological transition caused by the thermosensitivity of both the core-forming PHPMA block and the stabilizing P(GMA-stat-(MAm-GFF)) block.
Self-mineralization and assembly of a bis-silylated Phe–Phe pseudodipeptide to a structured bioorganic–inorganic material
Mater. Horiz., 2019, 6, 2040-2046 doi: 10.1039/C9MH00580C
Jebors S, Valot L, Echalier C, Legrand L, Mikhaleff R,Van Der Lee A, Arenal R, Dumy P, Amblard M, Martinez J, Mehdi A and Subra G.
Abstract
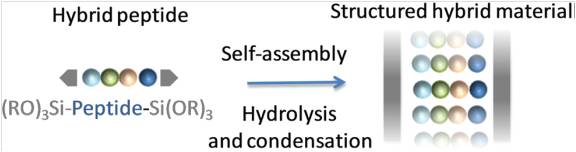
Self-mineralization of a trialkoxysilyl hybrid peptide yields in a single step a nanostructured hybrid material. A bis-silylated pseudodipeptide inspired from the Phe–Phe dipeptide was used to program the assembly by sol–gel polymerization under heterogeneous conditions, in water at pH 1.5 without any structure-directing agent. A mechanism deciphering the hybrid material assembly was proposed thanks to 1H NMR spectroscopy. First, water-insoluble hybrid building blocks were hydrolysed into their soluble silanol counterparts. Then, these transitional species, thanks to hydrogen bonding and π–π stacking, self-assembled in solution. Last, the proximity of the silanol moieties favoured their polycondensation into growing siloxane oligomers, which spontaneously precipitated to produce an ordered hybrid material.







